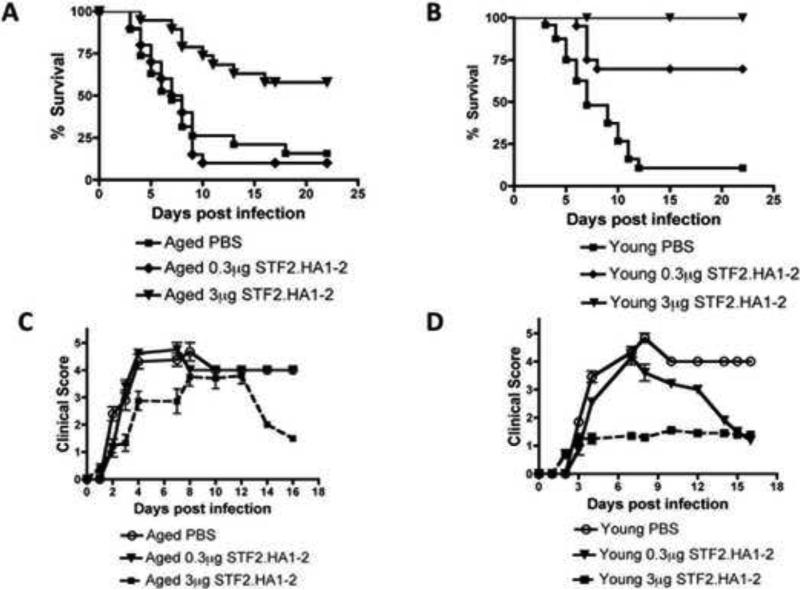
Figure 1. Effectiveness of STF2.HA1-2 vaccine to protect aged mice from subsequent influenza viral infection.
A: BALB/c aged mice were vaccinated with the 3μg or 0.3μg dose of STF2.HA1-2 vaccine according to protocol described in material and methods or PBS control and then i.n. challenged with influenza virus 1 week later. Mortality was recorded. Mice that received 3μg STF2.HA1-2 vaccine exhibited a significant extension of survival vs. the other groups (p<0.01, Logrank). N = 20 mice / group B: BALB/c young mice were vaccinated with the 3μg or 0.3μg dose of STF2.HA1-2 vaccine or PBS control and then challenged with influenza virus via i.n., injection 1 week later. Mortality was recorded. Mice that received either dose of STF2.HA1-2 exhibited a significant extension of survival vs. PBS control (p<0.01, Logrank). N = 20 mice / group C-D: Clinical score was recorded for vaccinated and control groups shown in A and B. Only the 3μg STF2.HA1-2 aged group exhibited an improved clinical status, whereas in young mice both vaccinated groups exhibited an improved clinical status although the 0.3μg STF2.HA1-2 group initially was sick and then recovered. N = 20 mice / group