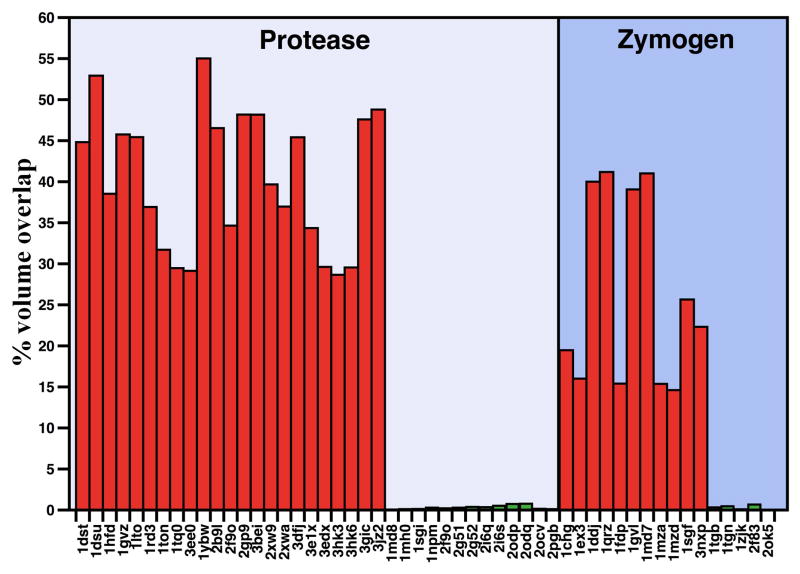
Figure 2.
Active site accessibility in the protease and zymogen. Accessibility of the active site for all proteases in the set (Box 1) was calculated in terms of the overlap with PPACK bound to the active site in the structure 1SHH of thrombin and expressed as percentage of the total volume of inhibitor. The structures in the set are all free of ligands bound to the active site or at sites known to affect activity or stability and therefore provide relevant sampling of the conformations accessible to the fold in the free form. The analysis identifies two groups in each set: one with considerable blockage of the active site where overlap with the volume to be occupied by PPACK is on the average 119 Å3 (36% of total, red bars), and the other with negligible or no overlap averaging 1.0 Å3 (0.3% of total, green bars). The dichotomous distribution supports existence of an equilibrium between mutually exclusive, collapsed and open forms in both the zymogen and protease.