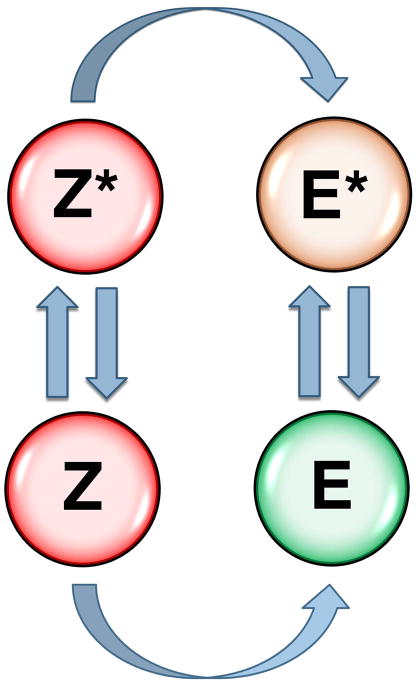
Figure 3.
Zymogen to protease conversion scheme. The classical zymogen to protease conversion scheme is extended to account for allostery in the zymogen and protease. Two forms of the zymogen, one with the active site open (Z) and the other with the active site collapsed (Z*) are in equilibrium and each converts irreversibly to the E or E* form of the protease. Both Z and Z* are inactive. Activity of the protease depends on the distribution between the inactive E* and active E form. The Z*-Z equilibrium in the zymogen controls the rate of conversion to the protease. Natural cofactors and synthetic molecules affect activity of the protease or the zymogen to protease conversion by altering the distribution of the various species in the scheme, as discussed in the text.