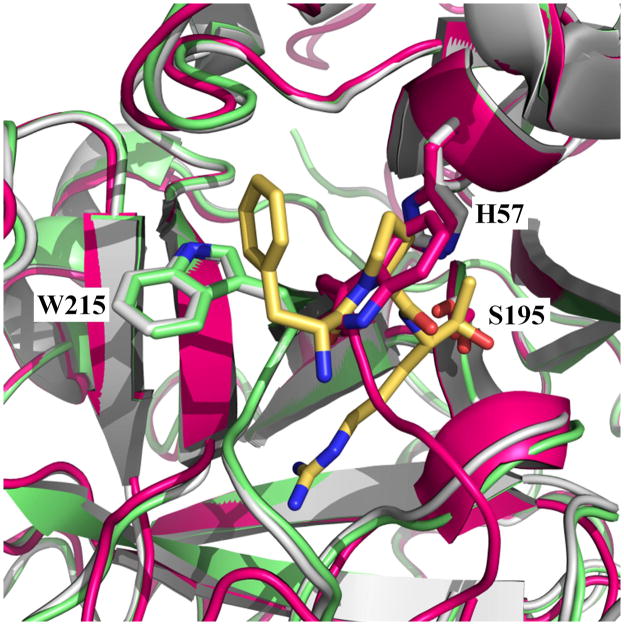
Figure I.
The E and E* forms. Overlay of the structures of thrombin in the E (1SGI, green) (35) and E* (3BEI, magenta) (38) form on the structure of thrombin bound to the active site inhibitor PPACK (1SHH, white) (35). PPACK (yellow) and residues H57, S195 and W215 from the protein are rendered as sticks. In the E form there is no overlap of residues 215-217 with the space occupied by PPACK in the active site. In the E* form, collapse of W215 and the 215-217 segment produces 159 Å3 occlusion of the space to be occupied by PPACK, which represents 48% of the total volume of the inhibitor (331 Å3).