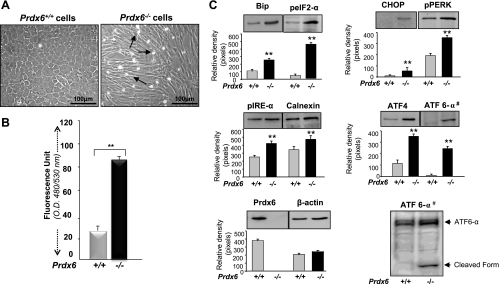
Fig. 1.
A: photomicrograph of Prdx6−/− cells cultured in vitro showing phenotypic abnormalities. Lens epithelial cells (LECs) were isolated from peroxiredoxin 6 (Prdx6)-targeted mutants (Prdx6−/−) and wild-type (Prdx6+/+) mice. Cells were cultured in complete DMEM medium (GIBCO) overnight. The medium was replaced with DMEM containing 0.1% BSA. Significant morphological alterations were observed in Prdx6−/− cells; they became elongated and fiberlike, formed cellular aggregates, packed irregularly, and showed apoptosis. Arrows indicate dead cells. B: involvement of oxidative stress in Prdx6−/− cells, showing elevated levels of reactive oxygen species (ROS). ROS-responsive fluorescence probe 2′,7′-dichlorofluorescein (H2-DCF-DA) assay was conducted to monitor the intracellular ROS level. Prdx6+/+ and Prdx6−/− cells were cultured in 96-well plates in DMEM + 10% FBS. The next day, the medium was replaced with HBSS containing H2-DCF-DA dye and fluorescence intensity was measured. Histogram values are means ± SD of three independent experiments. OD, optical density. **Statistically significant difference (P < 0.001 vs. control). C: Western analysis of Prdx6−/− and Prdx6+/+ cells showing expression or activation of Bip, CHOP, calnexin, peIF2-α, pPERK, pIRE-α, ATF4, and ATF6-α (#, nuclear extract) in Prdx6−/− and Prdx6+/+ cells. Cells were cultured in 60-mm plates for 48 h, cell lysates were prepared, proteins were resolved on 10% SDS-PAGE, and Western analysis was done. Membranes were stripped/restripped and immunostained with different antibodies. In each experiment, β-actin was used as an internal marker. Protein bands were quantified using a densitometer, and levels were normalized to corresponding β-actin levels; histograms are shown below the protein bands. Data represent means ± SD of three independent experiments. **P < 0.001 vs. control.