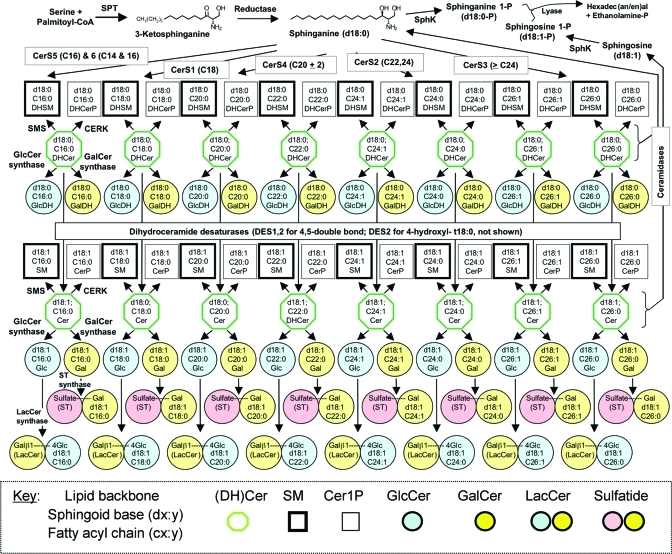
Figure 4.
De novo sphingolipid biosynthesis through lactosylceramide and sulfatide. Starting at top left, serine and palmitoyl-CoA are condensed by serine palmitoyltransferase (SPT) to form 3-ketosphinganine that is reduced to sphinganine, which is N-acylated by ceramide synthases (CerS) with the shown fatty acyl-CoA preferences, or phosphorylated by sphingosine kinase (SphK). The N-acylsphinganines (dihydroceramides, DHCer) can be incorporated into more complex dihydro-sphingomyelins, SM, from sphingomyelin synthases, SMS; -ceramide 1-phosphates, CerP, from ceramide kinase, CERK; -glucosylceramides, GlcCer, from GlcCer synthase; and -galactosylceramides, GalCer, from GalCer synthase). DHCer is also oxidized to Cer by dihydroceramide desaturase (DES1 and DES2; DES2 is also capable of hydroxylating the 4-position to form 4-hydroxydihydroceramides, t18:0) and incorporated into more complex sphingolipids as shown. The diagram also displays the formation of lactosylceramide (LacCer) from GlcCer and sulfatides (ST) from GalCer, and the turnover of DHCer to sphinganine (and Cer to sphingosine), which can be recycled or phosphorylated and cleaved to fatty aldehydes and ethanolamine phosphate. Not shown is ceramide phosphoethanolamine, which is present in mammalian cells in nearly trace amounts. The key is shown at the bottom, and is the same as for Figure 1 except that heavy black boxes represent SM, thin black for Cer1P, and (DH)Cer are represented by the green octagon.