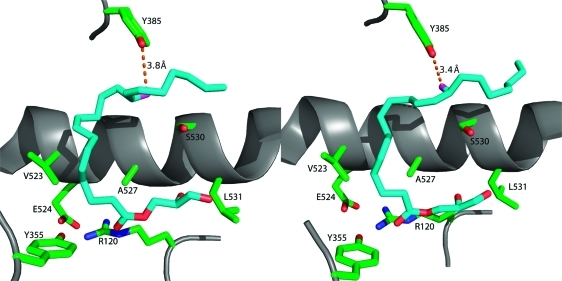
Figure 9.
Comparison of the conformations of 1-AG in the active sites of the two monomers of murine COX-2. The left frame displays 1-AG in an unproductive conformation in monomer A of the homodimer, whereas the right frame displays 1-AG bound in a productive conformation in monomer B of the homodimer. The two conformations are comparable, but the ω ends of the fatty acyl groups differ in conformation so that the 13-pro-(S) hydrogen is only close enough to Tyr-385 in monomer B to enable abstraction during the catalytic cycle.