Abstract
The Na-K-ATPase is part of a cell signaling complex, the Na-K-ATPase signalosome, which upon activation by the hormone ouabain regulates the function of different cell types. We previously showed that ouabain induces proliferation of epithelial cells derived from renal cysts of patients with autosomal dominant polycystic kidney disease (ADPKD cells). Here, we investigated the signaling pathways responsible for mediating the effects of ouabain in these cells. Incubation of ADPKD cells with ouabain, in concentrations similar to those found in blood, stimulated phosphorylation of the epidermal growth factor receptor (EGFR) and promoted its association to the Na-K-ATPase. In addition, ouabain activated the kinase Src, but not the related kinase Fyn. Tyrphostin AG1478 and PP2, inhibitors of EGFR and Src, respectively, blocked ouabain-dependent ADPKD cell proliferation. Treatment of ADPKD cells with ouabain also caused phosphorylation of the caveolar protein caveolin-1, and disruption of cell caveolae with methyl-β-cyclodextrin prevented Na-K-ATPase-EGFR interaction and ouabain-induced proliferation of the cells. Downstream effects of ouabain in ADPKD cells included activation of B-Raf and MEK and phosphorylation of the extracellular regulated kinase ERK, which translocated into the ADPKD cell nuclei. Finally, ouabain reduced expression of the cyclin-dependent kinase inhibitors p21 and p27, which are suppressors of cell proliferation. Different from ADPKD cells, ouabain showed no significant effect on B-Raf, p21, and p27 in normal human kidney epithelial cells. Altogether, these results identify intracellular pathways of ouabain-dependent Na-K-ATPase-mediated signaling in ADPKD cells, including EGFR-Src-B-Raf-MEK/ERK, and establish novel mechanisms involved in ADPKD cell proliferation.
Keywords: ion transport system
the na-k-atpase is a plasma membrane protein complex known to play two fundamental roles in the cell. On the one hand, it is the ion transport system that generates the Na+ and K+ gradients that typically exist across the cell plasma membrane (11). On the other hand, the Na-K-ATPase is the receptor for cardiotonic steroids, and the transducer that mediates the effects of ouabain and other endogenous digitalis-like compounds in the cell (3, 49). The Na-K-ATPase is composed of two major polypeptides, a catalytic α and a glycosylated β subunit (14). The α polypeptide represents the primary subunit involved in ouabain-dependent Na-K-ATPase signaling (5). Ouabain is a well-characterized hormone that is synthesized in the adrenal glands and is released into the circulation in response to several stimuli (34, 35). Once in the bloodstream, ouabain reaches the target cells, where it triggers a variety of cell responses, including changes in cell metabolism, motility, and growth (2, 8, 9, 15, 24, 25, 42).
At present, various components of the Na-K-ATPase signaling apparatus have been described. In myocardial and epithelial cells, the signaling Na-K-ATPase forms part of a protein complex that resides within the plasma membrane caveolae (18). Upon ouabain binding, the Na-K-ATPase initiates a series of reactions that include interaction with neighboring proteins, in what has been described as the Na-K-ATPase signalosome (31). This, in turn, leads to activation of the kinase Src and downstream members of the mitogen-activated protein kinase (MAPK) pathway (9, 13, 15, 45, 49). Previous studies in our laboratory showed that the Na-K-ATPase also plays a signaling role in cyst-lining renal epithelial cells from patients with autosomal dominant polycystic kidney disease (ADPKD cells). In these cells, ouabain, in concentrations similar to those circulating in blood, stimulates ADPKD cell mitosis and growth (27).
ADPKD is the most common inherited disease of the kidney, characterized by the formation and progressive expansion of multiple fluid-filled cysts that distort the structure and severely compromise the function of the organ (12). Aberrant proliferation of the renal epithelial cells is one of the hallmarks of ADPKD, and it represents an essential event in the development of renal cystogenesis (7, 29, 37). Although ADPKD has a genetic origin, identified as alterations in the Pkd1 and Pkd2 genes that encode for polycystin 1 and 2 (PC1 and PC2), respectively, the progressive enlargement of cysts appears to be regulated by a variety of nongenetic factors (40, 47). Various pharmacological and physiological agents have been shown to stimulate ADPKD cystogenesis. For example, arginine vasopressin, a cAMP agonist, and epidermal growth factor (EGF) stimulate cell proliferation of human ADPKD cells through activation of the mitogen-activated kinase-extracellular regulated kinase (MEK-ERK) pathway; and cAMP promotes the proliferation of human ADPKD cells and accelerates cyst growth in animal models of polycystic kidney disease (43). The cAMP-dependent cell proliferation is mediated by activation of B-Raf, a kinase that phosphorylates and stimulates MEK (7, 50). In contrast, B-Raf is repressed in normal renal cells and cAMP is unable to stimulate ERK and cell proliferation. Thus, B-Raf appears to be an important intermediate in the activation of ERK and proliferation of cyst epithelial cells (43).
Because of its mitogenic action, ouabain emerges as a factor capable of accelerating renal cystic epithelial growth. At present, the pathways involved in ouabain-induced and Na-K-ATPase-mediated effects in ADPKD cells are unknown. Deciphering the intermediate molecules through which ouabain exerts its action in ADPKD is important in understanding the mechanisms underlying cyst formation and progression of the disease. In the present study, we investigated the signaling events triggered by physiological concentrations of ouabain in ADPKD cells. We show that ouabain stimulation of proliferation of the cystic cells requires the integrity of caveolae, the EGF receptor (EGFR), and Src. In addition, ouabain-dependent Na-K-ATPase signaling involves downstream activation of members of the MAPK pathway, translocation of ERK to the cell nucleus, and downregulation of the expression of the cyclin-dependent kinase inhibitors p21 and p27.
MATERIALS AND METHODS
Cell culture.
Primary cell cultures were derived from surface cysts of kidneys from patients with ADPKD. The cells, obtained from nephrectomy specimens, were generated by the PKD Biomaterial Core at University of Kansas Medical Center. A protocol for the use of discarded human kidney tissues was approved by the Institutional Review Board at University of Kansas Medical Center. Primary cultures were prepared as described (44). Cells were seeded and grown in DME/F12 supplemented with 5% heat-inactivated FBS, penicillin/streptomycin, 5 μg/ml insulin, 5 μg/ml transferrin, and 5 ng/ml sodium selenite (ITS). In some experiments, normal human kidney cell cultures were used and prepared as described (44). Treatment of the cells with ouabain was performed at a final concentration of 3 nM, using incubation times of 24 h, for determination of cell proliferation, or 30 min, to study activation of intracellular mediators of Na-K-ATPase signaling pathway. Other treatments included addition of the EGFR inhibitor tyrphostin AG1478 and the Src inhibitor PP2, which were used at concentrations of 2 and 10 μM, respectively.
Cell proliferation assays.
Measurement of cell proliferation was performed as described (27). Briefly, ADPKD cells (4,000 cells/well) were seeded onto a 96-well plate with culture medium supplemented with 1% FBS and ITS. After 24 h, the medium was aspirated and replaced by medium without ITS and with a reduced amount of serum (0.002%). After an additional 24 h, the cells were treated without and with 3 nM ouabain for 24 h. This concentration of ouabain is within the physiological levels found in blood and induces maximal proliferation in ADPKD cells (27). Cell proliferation was determined using the Promega CellTiter 96 MTT Assay according to the manufacturer's recommendations (Promega, Madison, WI). This assay provides adequate estimates of ADPKD cell proliferation, as previously validated through comparisons with direct counting of the cells (27).
Reverse transcriptase-polymerase chain reaction.
Total RNA from each cell type was isolated using TRIzol reagent according to the supplier specifications (Invitrogen, Carlsbad, CA). Complementary DNA was generated by reverse transcription using the SuperScript First-Strand Synthesis System (Invitrogen) and oligo (dT) primers as described (27). The resulting first-strand cDNA was amplified using primers specific to the cyclin kinase inhibitors p21 and p27. The conditions for PCR included a first cycle of 30 s at 94°C, followed by 30 cycles at 94°C for 15 s, 60°C for 15 s, and 72°C for 30 s. The amplified DNA fragments were identified by electrophoresis in a 1% agarose gel stained with ethidium bromide.
Immunoblot analysis.
ADPKD cells (1 × 105) were grown in six-well plates and were treated with and without 3 nM ouabain for 30 min and in the absence and presence of the indicated inhibitors. Cells were washed with ice-cold phosphate saline (PBS) and lysed with lysis buffer containing 1% NP-40, 0.25% sodium deoxycholate, 1 mM sodium orthovanadate, 1 mM NaF, 150 mM NaCl, 1 mM EDTA, 50 mM Tris, and 1× protease inhibitor cocktail (Sigma, St. Louis, MO). Total protein content in the cleared lysates was determined using the dye-binding assay from Bio-Rad (Hercules, CA). Equal amounts of total protein (15 μg) were separated by 10% SDS-PAGE and transferred to nitrocellulose membranes (NitroBind, GE Water and Process Technologies, Trevose, PA). Immunoblots were first analyzed for levels of the phosphorylated forms of the proteins of interest, and then the membranes were stripped and probed to also include the nonphosphorylated or total forms of the polypeptides. The antibodies used were anti-total Src and anti-Src[pY-418] from Biosource-Invitrogen (Faraday, CA), anti-total EGFR and anti-EGFR[pY-1173] from Upstate Cell Signaling (Lake Placid, NY), anti-total caveolin and anti-caveolin [pY-14] from BD Biosciences (San Jose, CA), and anti-total ERK, anti-ERK[pY-204], anti-total-B-Raf, and anti-B-Raf [pT-598/ pS-601] from Santa Cruz Biotechnology (Santa Cruz, CA). Horseradish peroxidase-conjugated secondary antibodies and chemiluminescence were used for detection. The images were scanned and quantified for band intensity using the Gel-Pro software (Media Cybernetics, Silver Spring, MD). The level of each phosphorylated cell intermediary was determined as the ratio of the phosphorylated to total forms of the proteins, and expressed in relative density units, relative to the untreated controls.
Immunocytochemistry.
ADPKD cells were grown on 18-mm glass coverslips in 12-well tissue culture plates. After treatment with or without 3 nM ouabain for 24 h, cells were fixed in 4% paraformaldehyde and samples were processed for immunocytochemistry as described (27). Briefly, cells were permeabilized with 0.3% Triton X-100 in 25 mM HEPES, pH 7.4, 150 mM NaCl, and 1 mM EGTA (HBS). After being blocked for 2 h at room temperature with 0.2% BSA and 2% normal goat serum in HBS, the primary antibodies were applied. Following overnight incubation at 4°C, samples were washed three times, 15 min each, and were treated with the secondary antiserum. As secondary antibodies, FITC-conjugated antibodies were used for B-Raf and ERK, respectively. After being washed, samples were mounted on slides using Prolong gold antifade reagent (Molecular Probes-Invitrogen). B-Raf samples were observed under fluorescent microscopy, while for ERK, confocal microscopy was used.
Immunoprecipitation assays.
Immunoprecipitation was used to determine the ouabain-dependent association of the Na-K-ATPase with EGFR. ADPKD cells were grown in six-well plates and were treated with 3 nM ouabain for 30 min. Cells were lysed in the lysis buffer described above and were subjected to immunoprecipitation as described (16). The anti-EGFR antibody and magnetic beads coated with secondary anti-rabbit antibody were used to pull down the polypeptide. After overnight incubation on a rocking surface at 4°C, the beads were isolated with a magnet and washed three times in the lysis buffer. The precipitated proteins were eluted in sample buffer (100 mM Tris·HCl, pH 6.8, 2% SDS, 33% glycerol, 100 mM DTT) for 15 min at 65°C, and they were separated by SDS-PAGE (7.5% gel). Proteins were transferred to nitrocellulose and immunobloted for detection of the Na-K-ATPase α-subunit, using the anti-α C464/6B antibody generously provided by Michael Caplan (Yale University). Immunoprecipitation was also used to determine phosphorylation of the kinase Fyn. Basically, the same procedure was followed, but for this, anti-Fyn antibody (Santa Cruz Biotechnology) was used for precipitation of Fyn, while anti-phospho tyrosine clone 28 antibody (Biosource International, Camarillo, CA) was used to detect the active phosphorylated Fyn.
Cholesterol depletion and repletion.
Cholesterol depletion was obtained by treating the cells with 10 mM methyl-β-cyclodextrin (methyl-β-CD) for 30 min at 37°C as previously described (45). Cells were washed twice in culture medium and incubated with or without 3 nM ouabain, in the presence of 0.5 mM methyl-β-CD. Cells were then analyzed for proliferation, after 24 h, or for determination of Na-K-ATPase-EGFR association, after 30 min. In the cholesterol repletion experiments, cells that had been depleted of cholesterol were treated in the culture medium with 40 μl/ml of cholesterol/methyl-β-CD stock solution as described (45). The cholesterol/methyl-β-CD solution was prepared by mixing 200 μl of a 20-mg/ml solution of cholesterol in ethanol with 10 ml of 10% methyl-β-CD.
Kinase activity assays.
Cells were treated with and without 3 nM ouabain for 30 min. Cells were then washed with ice-cold PBS and lysed with RIPA buffer containing 1% NP-40, 0.25% sodium deoxycholate, 1 mM sodium orthovanadate, 1 mM NaF, 150 mM NaCl, 1 mM EDTA, 50 mM Tris, and 1× protease inhibitor cocktail (Sigma). After total protein content determination using the dye-binding assay from Bio-Rad, lysates were subjected to immunoprecipitation using an anti-Src antibody (Biosource-Invitrogen) and protein G agarose beads (Santa Cruz Biotechnology). The immunoprecipitation mixture was incubated overnight at 4°C on a rocking table. The immunoprecipitate was then washed three times with ice-cold PBS and the pulled-down proteins were used to measure Src or B-Raf kinase activity. For Src, samples were resuspended in reaction buffer containing 100 mM Tris·HCl (pH 7.2), 125 mM MgCl2, 5 mM MnCl2, 2 mM EGTA, 250 μM sodium orthovanadata, and 2 mM dithiothreitol. The Src kinase reaction was performed with a commercially available kit (Upstate Biotechnology, Lake Placid, NY) following the manufacturer's instructions. For the B-Raf, reaction buffer containing 20 mM MOPS (pH 7.2), 25 mM β-glycerol phosphate, 5 mM EGTA, 1 mM sodium orthovanadate, and 1 mM dithiothreitol was used. The B-Raf kinase reaction was performed with a commercially available kit from Millipore (Temecula, CA), following the manufacturer's instructions. For both Src and B-Raf, reactions were carried out with [γ-32P]-ATP and the phosphorylation of a Src, or B-Raf substrate peptide, which was provided with the manufacturer's kit, was measured in a scintillation counter.
Nuclear fraction preparations.
Nuclear extracts were prepared for determination of p21 and p27 using the CNM Compartment extraction kit (Biochain Institute, Hayward, CA), following the maufacturer's instructions. Briefly, ADPKD cells were treated in the absence or presence of 3 nM ouabain for 30 min in 100-mm Petri culture dishes and rinsed with PBS. Then, 400 μl of ice-cold buffer C from the kit were added and cells were scraped. The resulting cell lysate was mixed for 20 min and centrifuged at 11,000 g at 4°C. The pellet was resuspended in 150 μl of buffer N, in the presence of protease inhibitors. Samples were mixed for 20 min and centrifuged at 11,000 g. The resulting supernatant, containing the nuclear fraction, was collected. Samples were subjected to immunoblot using anti-p21 and anti-p27 antibodies (Santa Cruz Biotechnology) and anti-histone 3 from Cell Signaling Technology (Danvers, MA).
Data analysis.
Statistical significance of the differences between ouabain-treated and -untreated controls was determined by Student's t-test. Statistical significance was defined as P < 0.05.
RESULTS
Ouabain-stimulated proliferation of human ADPKD cells involves the kinase Src and EGFR.
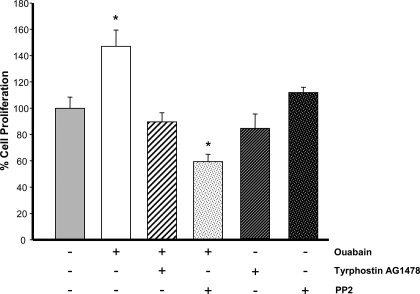
We previously demonstrated that physiological concentrations of ouabain, acting through the Na-K-ATPase, stimulate the proliferation of human ADPKD cells (27). At present, the mitogenic pathways triggered by ouabain in these cells are unknown. In other cell types, ouabain binding to the Na-K-ATPase has been shown to convert it into a functional receptor tyrosine kinase that leads to Src kinase activation. This induces the secondary tyrosine phosphorylation of a number of proteins including EGFR (31). To determine whether the proliferation of ADPKD cells induced by ouabain involves the EGFR and Src, the effect of inhibitors of Src and EGFR was explored. Cells were treated in the absence and presence of 3 nM ouabain, for 24 h and without or with the EGFR blocker tyrphostin AG1478, or the Src kinase inhibitor PP2 {4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine}. As previously described, the amounts of ouabain and incubation time are optimal for determination of the effects of this hormone on proliferation of ADPKD cells (27). Concentrations of the inhibitors were based on those previously used in ADPKD cells (50, 51). As shown in Fig. 1, ouabain induced growth of the ADPKD cells. This agrees with our previous findings indicating that ouabain stimulated ADPKD cell division and mitotic index (27). The proliferative effect of ouabain was prevented by tyrphostin AG1478 and PP2 inhibitors. Both of these inhibitors did not have significant effects on ADPKD cell proliferation when used alone (Fig. 1). These results suggest the involvement of EGFR and Src kinase in mediating ouabain effects in ADPKD cell proliferation.
Fig. 1.
Ouabain-induced proliferation of autosomal dominant polycystic kidney disease (ADPKD) cells requires epidermal growth factor receptor (EGFR) and Src. Cells grown in 96-well plates were treated for 24 h without and with 3 nM ouabain in the absence and presence of 2 μM tyrphostin AG1478, or 10 μM PP2, or with just these two inhibitors alone. ADPKD cell proliferation was determined using the CellTiter 96 MTT Assay. Bars represent means ± SE of 8 experiments performed on cells from 8 different ADPKD kidneys. Values were normalized to the untreated control. *P < 0.001 compared with untreated controls.
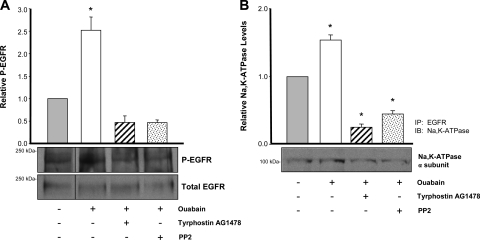
An early event after ouabain binding to the Na-K-ATPase of other cell types is phosphorylation of the EGFR (15). To determine whether ouabain leads to EGFR phosphorylation in ADPKD cells, we measured levels of phosphorylated EGFR before and after addition of 3 nM ouabain for 30 min. As previously shown, this incubation time with ouabain causes a maximal stimulation of ERK in these cells (27). This was performed in the absence and presence of PP2 and tyrphostin AG1478. Immunoblot analysis of cell lysates was performed using antibodies specific to the total and phosphorylated forms of EGFR and the phosphorylated EGFR (P-EGFR)-to-total EGFR ratios were determined. Consistent with an involvement of EGFR in Na-K-ATPase signaling in ADPKD cells, phosphorylated EGFR amounts significantly increased in the presence of ouabain (Fig. 2A). Moreover, treatment of the cells with PP2 and tyrphostin AG1478 abrogated the ouabain-dependent phosphorylation of EGFR (Fig. 2A).
Fig. 2.
Ouabain-induced Na-K-ATPase signaling involves the EGFR in ADPKD cells. A: ouabain-induced phosphorylation of EGFR. Cells were treated with or without 3 nM ouabain, either alone, or in the presence of tyrphostin AG1478 for 30 min. Cell lysates were subjected to immunoprecipitation, using a polyclonal anti-EGFR as the pull-down antibody. Immunoprecipitated proteins were separated in 8% SDS-PAGE and immunoblotted. Blots were first probed with an anti-phosphotyrosine antibody to detect phosphorylated EGFR (P-EGFR) and then stripped and reprobed with an anti-EGFR antibody. Bars represent the relative phosphorylated levels of EGFR (P-EGFR/total EGFR), presented as a ratio of the untreated controls of 4 determinations performed on cells from 4 different ADPKD kidneys. *P < 0.001 compared with the untreated control. B: interaction of the Na-K-ATPase with EGFR. Cells were treated as in A and cell lysates were immunoprecipitated with a polyclonal anti-EGFR antibody (IP: EGFR). Immunoprecipitated proteins were subjected to 10% SDS-PAGE and immunoblotted using a monoclonal antibody against the α1-subunit of the Na-K-ATPase, C464/6B (IB: Na-K-ATPase). Values represent means ± SE of 3 determinations on cells from 3 different ADPKD kidneys. *P < 0.01 compared with the untreated control.
To further explore the involvement of EGFR in ouabain-dependent Na-K-ATPase signaling of ADPKD cells, we determined whether ouabain caused association of the Na-K-ATPase with EGFR, using immunoprecipitation assays. ADPKD cells were treated without and with 3 nM ouabain alone, or in the presence of PP2 and tyrphostin AG1478 for 30 min. Then, cells were lysed and EGFR was immunoprecipitated. The pulled down proteins were subjected to immunoblots to determine the presence of the α-subunit of the Na-K-ATPase. As shown in Fig. 2B, the Na-K-ATPase catalytic polypeptide coimmunoprecipitated with the EGFR. Importantly, association between both polypeptides was increased by ouabain and was reduced in the presence of the Src and EGFR inhibitors. Altogether, these results suggest that EGFR is an intermediate in the Na-K-ATPase signaling cascade that transduces ouabain effects in ADPKD cells.
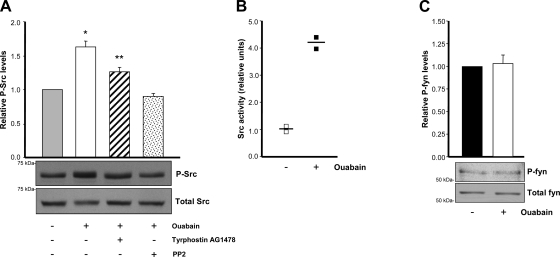
To determine the involvement of Src in ouabain-dependent Na-K-ATPase signaling in ADPKD cells, we next measured Src phosphorylation, following experiments similar to those described for the EGFR. After treatment of the cells for 30 min, with and without 3 nM ouabain and PP2 or tyrphostin AG1478, cell lysates were subjected to immunoblot analysis, using antibodies specific to the total and phosphorylated forms of Src. Amounts of phosphorylated Src (P-Src) significantly increased in the presence of ouabain, and this effect was prevented by PP2 and tyrphostin AG1478 (Fig. 3A). The anti-phospho-Src antibody recognizes phosphorylation of Tyr 418 in the protein, which is the residue modified during Src activation. To further confirm activation of Src, we directly measured Src kinase activity before and after ouabain addition. Higher values for Src activity were determined after ouabain treatment in ADPKD cells (Fig. 3B). In contrast to the effect on Src, ouabain did not change the phosphorylated levels of Fyn, another member of the Src family type of kinases (Fig. 3C). These results show that Src kinase is involved in the response of ADPKD cells to ouabain and suggest that this effect is relatively specific, not extending to all Src type of kinases.
Fig. 3.
Ouabain effects are mediated through Src in ADPKD cells. A: phosphorylation of Src. Cells were treated with or without 3 nM ouabain, either alone, or in the presence of tyrphostin AG1478 for 30 min. Lysates from the cells were subjected to immunoblot and phosphorylated Src was detected using an antibody against the activated Src (anti-Src-Y418 antibody). Blots were then stripped and reimmunoblotted with an anti-total Src. Bars represent the relative phosphorylated amounts of Src, P-Src/Total Src, presented as a ratio of the untreated controls of 4 determinations performed on cells from 4 different ADPKD kidneys. Values different from the untreated controls are shown; *P < 0.001 and **P < 0.01. B: Src kinase activity. Cells treated in the absence and presence of ouabain were lysed and Src activity was measured using a method based on the incorporation of [γ-32P] from [γ-32P]-ATP into a Src-specific substrate peptide. Symbols represent data from 2 experiments and the means performed on cells from 2 different ADPKD kidneys. C: phosphorylation of Fyn. Cells were treated with or without 3 nM ouabain, lysed, and subjected to immunoprecipitation with anti-total Fyn antibody. After immunoblot, phosphorylated Fyn was detected using anti-phospho Fyn antibody. Total Fyn was determined in the imput material before immunoprecipitation. Bars represent the relative phosphorylated Fyn, P-Fyn/Total Fyn, presented as a ratio of the untreated controls.
Ouabain-dependent Na-K-ATPase signaling in ADPKD cells requires intact cell caveolae and phosphorylation of caveolin 1.
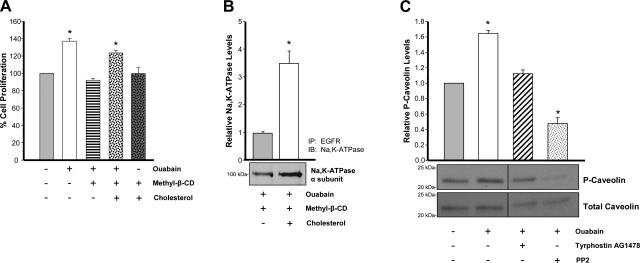
It has been reported that two pools of Na-K-ATPase exist in cells and that the population involved in signaling is confined to the microdomains of cholesterol-containing lipid rafts of the plasma membrane caveolae (45). To explore the role of caveolae in ouabain-induced effects in ADPKD cells, we investigated the dependence of Na-KATPase signaling on caveolae by disrupting these membrane structures in the cells through cholesterol depletion with methylβ-CD. This method has been commonly used to study caveolar function (45). As a first approach, we studied the effect of methyl-β-CD on the ouabain-stimulated proliferation of ADPKD cells, determined after 24 h. Figure 4A shows that treatment of ADPKD cells in the culture medium with methyl-β-CD prevented the increase in cell number caused by ouabain. The requirement of caveolae for Na-K-ATPase signaling in the cells was confirmed by replenishing cholesterol, a technique that has been frequently used for this purpose (45). Addition of cholesterol was able to revert the effect of methyl-β-CD in ouabain-induced ADPKD cell growth (Fig. 4A, fourth bar). Importantly, methyl-β-CD and cholesterol alone did not affect ADPKD cell proliferation (Fig. 4A, last bar). Another indication that Na-K-ATPase signaling requires normal function of cell caveolae was obtained by studying the effect of methyl-β-CD in the association of the Na-K-ATPase with the EGFR. For this, after treatment with ouabain and methyl-β-CD for 30 min, cells were subjected to immunoprecipitation assays. Cholesterol depletion blocked the ouabain-dependent interaction of the Na-K-ATPase α-subunit with EGFR, and returning cholesterol back to the cell culture media was able to offset the effect of methyl-β-CD, recovering Na-K-ATPase-EGFR association (Fig. 4B). Altogether, these results suggests that Na-K-ATPase signaling in ADPKD cells depends on the presence of caveolar domains at the plasma membrane of the cells.
Fig. 4.
Ouabain signaling requires caveolae and involves caveolin 1 in ADPKD cells. A and B: cholesterol depletion with methyl-β-cyclodextrin. ADPKD cell cultures were treated for 30 min with 10 mM methyl-β-CD at 37°C to deplete cholesterol and disrupt cell caveolae. Cells were then washed twice in serum-free media and exposed to 3 nM ouabain and 0.5 mM methyl-β-cyclodextrin to maintain a cholesterol-free environment. In some cases, cholesterol was added back to the cultures to replenish the cells with this sterol. After these treatments, cells were treated with 3 nM ouabain for 24 h to analyze ouabain-induced cell proliferation. B: experiments were performed as described in A, except that treatment with ouabain was for 30 min before analyzing the association of the Na-K-ATPase with EGFR. Bars represent means ± SE of 3 determinations on cells from 3 ADPKD kidneys. Significant differences from untreated controls and/or cultures where cholesterol was depleted are shown, with *P < 0.01. C: phosphorylation of caveolin-1. Cells were treated with 3 nM ouabain for 30 min in the absence or presence of tyrphostin AG1478, or the Src inhibitor, PP2. Cleared lysates were subjected to 15% SDS-PAGE and immunoblot, using a monoclonal anti-caveolin antibody against Tyr14. Blots were then stripped and reprobed with a total polyclonal anti-caveolin antibody. Bars represent the relative phosphorylated amounts of caveolin-1 (P-caveolin/total caveolin), presented as a ratio of the untreated controls. Values are means ± SE from 3 determinations on cells from 3 ADPKD kidneys. Statistically significant differences compared with untreated control are shown, with *P < 0.01.
A major protein component of caveolae are the caveolins, a family of membrane-associated scaffolding proteins intimately involved in a variety of cellular processes including signal transduction, endocytosis, and tumorigenesis (32, 36). Caveolin-1 in particular has been shown to be involved in Na-K-ATPase-mediated signaling (49). To determine whether the Na-K-ATPase signaling complex in ADPKD cells involves caveolin 1, we explored the ability of ouabain to stimulate phosphorylation of caveolin 1. For this, after treatment with or without 3 nM ouabain for 30 min, cells were lysed and subjected to immunoblots for analysis of phosphorylated caveolin 1 (P-Caveolin). As presented in Fig. 4C, levels of P-Caveolin relative to total caveolin significantly increased with ouabain. The incorporation of phosphate into caveolin required activation of Src, but not the EGFR. Thus, addition of PP2, but not tyrphostin AG1478, interfered with formation of P-Caveolin in the cells (Fig. 4C). This set of experiments supports the role of caveolae in ouabain-mediated Na-KATPase signaling of ADPKD cells, and it suggests a direct involvement of caveolin 1 in the process.
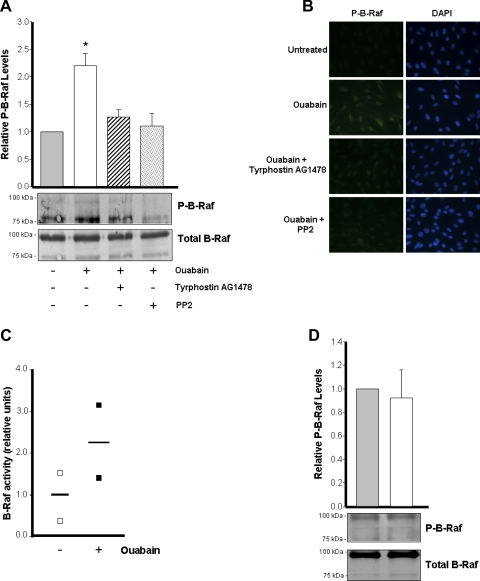
Ouabain-dependent Na-K-ATPase signaling of ADPKD cells involves activation of B-Raf.
Ouabain effects have been shown to include the phosphorylation of several cell kinases (49). Because of the importance of the kinase B-Raf in ADPKD cells (50), we investigated the role of B-Raf in ouabain-induced signaling. ADPKD cells were treated with or without 3 nM ouabain for 30 min in the presence and absence of tyrphostin AG1478 and PP2, and phosphorylation of B-Raf was explored by immunoblot. As shown in Fig. 5A, ouabain increased the level of phosphorylated B-Raf (P-B-Raf). B-Raf was detected as two bands and both were included when measuring the total and phosphorylated forms of this kinase. From both of the B-Raf bands detected, the anti-phospho B-Raf antibody labeled more apparently the band with lower molecular weight, suggesting that this form of B-Raf is the primary one phosphorylated in ADPKD cells. Inhibition of Src and EGFR, which are essential for Na-K-ATPase signaling, precluded B-Raf phosphorylation (Fig. 5A). Similar results were obtained through immunofluorescence analysis (Fig. 5B). In addition, after addition of ouabain to the cells, a trend for an increase in B-Raf activity was observed (Fig. 5C). In contrast, the levels of phosphorylated B-Raf were not significantly different before and after ouabain treatment in normal kidney cells. Altogether, these results suggest that B-Raf is a mediator of ouabain-induced Na-K-ATPase signaling in ADPKD cells and that B-Raf is downstream of EGFR and Src.
Fig. 5.
Ouabain effects are mediated through B-Raf in ADPKD cells. A and B: phosphorylation of B-Raf. Cells were treated without and with 3 nM ouabain for 30 min in the absence and presence of tyrphostin AG1478 and PP2. Phosphorylation of B-Raf in ADPKD was determined by immunoblot analysis (A) and immunofluorescence microscopy (B). A: bars represent the relative levels of phosphorylated B-Raf (P-B-Raf/total B-Raf), presented as means ± SE of the ratio of the untreated controls, obtained from 5 determinations, using cells from different ADPKD kidneys. Significant differences are shown with *P < 0.01 compared with untreated control. C: BRaf kinase activity. Cells treated in the absence and presence of ouabain were lysed and B-Raf activity was measured using a method based on the incorporation of [γ-32P] from [γ-32P]-ATP into a B-Raf-specific substrate peptide. Symbols represent the values and horizontal bars are the mean of 2 separate determinations. D: phosphorylation of B-Raf in normal human kidney cells. Cells were treated without and with 3 nM ouabain for 30 min. Phosphorylation of B-Raf was determined as for the ADPKD cells. Bars represent the relative levels of phosphorylated B-Raf (P-B-Raf/total B-Raf), presented as means ± SE of the ratio of the untreated controls, obtained from triplicate determinations.
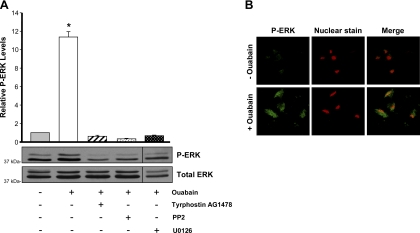
Ouabain-dependent Na-K-ATPase signaling requires phosphorylation of ERK in ADPKD cells.
A key kinase in the ouabain-activated cell signaling pathway is ERK (9). Our previous observations showed that activation of ERK is a response of ADPKD cells to physiological doses of ouabain (27). To further study the involvement of ERK, and evaluate this event in the context of other mediators of ouabain signaling, we determined ouabain-induced phospho-ERK (P-ERK) formation in ADPKD cells in the presence of various cell signaling inhibitors. As shown in Fig. 6A, treatment with 3 nM ouabain for 30 min stimulated ERK phosphorylation in ADPKD cells. Addition of the MEK inhibitor U0126 blocked ouabain-dependent phosphorylation of ERK. Moreover, treatment with PP2 and tyrphostin AG1478 also prevented ouabain-induced phosphorylation of ERK (Fig. 6A). These results indicate that, in ADPKD cells, ERK is an intermediate of the ouabain-dependent Na-K-ATPase signaling pathway and that its activation is dependent on the upstream mediators Src and EGFR.
Fig. 6.
Ouabain stimulates ERK phosphorylation via the EGFR-Src-MEK pathway in ADPKD cells. A: ouabain-dependent ERK phosphorylation levels. Cells were treated for 30 min without or with 3 nM ouabain alone or with inhibition of the EGFR (AG1478), Src (PP2), or MEK (U0126). Lysates from the cells were subjected to immunoblot to determine the phosphorylated and total forms of ERK. Bars represent the ratio of P-ERK/ERK, relative to the untreated controls, presented as means ± SE of 12 determinations performed on cells obtained from 8 ADPKD kidneys. Statistical significant differences compared with untreated controls are shown; *P < 0.001. B: ouabain-dependent localization of P-ERK. ADPKD cells grown on glass coverslips were treated without or with 3 nM ouabain for 30 min and subjected to immunocytochemistry and confocal microscopy. Cells were labeled with an anti-phospho-ERK antibody, followed by FITC-conjugated secondary antibody. Cell nuclei were stained with ethidium homodimer.
In addition, we studied ouabain stimulation of ERK phosphorylation using immunocytochemisty and confocal microscopy. This approach also showed that ouabain increased P-ERK levels in cyst epithelial cells. Moreover, P-ERK was found to be localized both to the cytoplasm and the nucleus of ADPKD cells (Fig. 6B). This further supports our hypothesis that ERK is a mediator in the ouabain signal transduction pathway involved in nuclear activation of genes responsible for the proliferation of the cells.
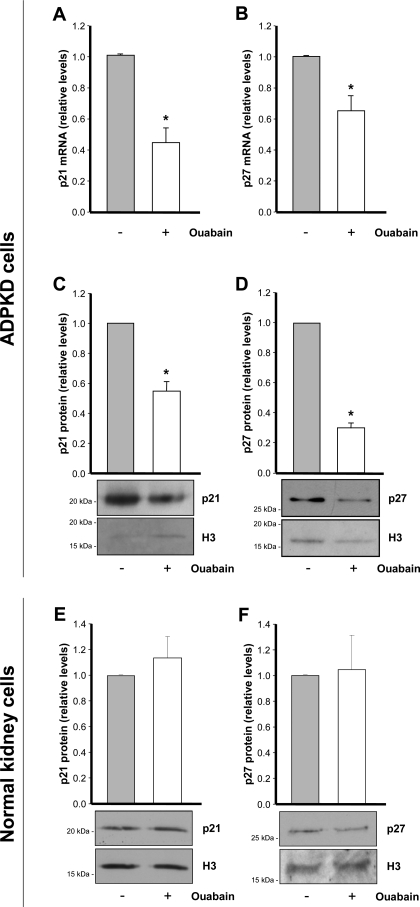
Ouabain downregulates expression of cyclin-dependent kinase inhibitors in ADPKD cells.
Ultimately, the effect of ouabain on cell growth may depend on regulation of proteins involved in the cell cycle. Progression through the cell cycle is tightly controlled by a group of proteins known as cell cycle regulatory proteins (22, 23). Among these proteins, the cyclin-dependent kinase inhibitors, or CKI, are central controllers of cell proliferation and differentiation (17). Within the CKI proteins, p21 and p27 have been shown to play a critical role in ADPKD (4, 28, 30). Therefore, to determine whether the ouabain-dependent growth of ADPKD cells depends on regulation of CKI proteins, we measured the levels of p21 and p27 mRNA and protein, before and after addition of 3 nM ouabain for 24 h. Treatment with ouabain caused a significant decrease in p21 and p27 mRNA (Fig. 7, A and B). This was correlated with a reduction of ∼40 and 70% in p21 and p27 proteins, respectively (Fig. 7, C and D). Instead, ouabain did not cause significant changes in the levels of p21 and p27 in normal human kidney cells (Fig. 7, E and F). These results show that ouabain can influence expression of p21 and p27 cell cycle inhibitors in ADPKD cells and that this effect is consistent with the stimulation that ouabain produces in proliferation of these cells.
Fig. 7.
Ouabain inhibits expression of p21 and p27 in ADPKD cells, but not in normal human kidney cells. Cells were treated without or with 3 nM ouabain and were incubated for 24 h. A–D: ADPKD cells. A and B: RNA was isolated from the cells, subjected to RT-PCR for detection of p21 and p27 transcripts. C and D: nuclear fractions from the cells were prepared and subjected to immunoblot for quantification of p21 and p27 protein levels. E and F: p21 and p27 levels in nuclear fractions from normal kidney cells. C–F: detection of histone 3 (H3) was included as a loading control. Bars represent the level of protein expression after densitometric analysis of the blots. Values are means ± SE of 3 experiments using cells from 3 different ADPKD or normal kidneys. Significant differences compared with the untreated controls are shown; *P < 0.01.
DISCUSSION
Studying the mechanisms of action of ouabain is a topic of general biological importance. Understanding how ouabain induces mitosis and proliferation of ADPKD cells is of particular interest since this may increase cyst formation and enlargement. In the present work, we explored the intracellular events involved in mediating ouabain-induced proliferation of human ADPKD cells. Our results show that ouabain-dependent Na-K-ATPase signaling in ADPKD cells requires plasma membrane caveolae and phosphorylation of the caveolar protein, caveolin 1. In this manner, similar to normal cells (45), ADPKD cells conserve the ability of transducing ouabain effects through the Na-K-ATPase localized in the cholesterol-enriched lipid raft compartment of caveolae. Our previous results showed that ADPKD cells exhibit an exacerbated response to ouabain and an affinity for this cardiotonic steroid that is higher than that of normal human kidney cells (27). Only a fraction of the Na-K-ATPase of ADPKD cells can respond to nanomolar concentrations of ouabain. This Na-K-ATPase population corresponds to ∼25% of the total Na-K-ATPase of the ADPKD cells (27). It is conceivable that this Na-K-ATPase population that responds to relative low-ouabain concentrations, such as those used in our study, may correspond to the signaling Na-K-ATPase specifically confined to the cell membrane caveolae. Although further experiments will be necessary to prove this possibility, our results indicate that ouabain-induced signaling in ADPKD cells depends on Na-K-ATPase associated with caveolae.
Our results also demonstrate that early events in the ouabain-dependent Na-K-ATPase signaling in ADPKD cells involve EGFR and Src. Thus, ouabain induces phosphorylation of EGFR, association of this protein with the Na-K-ATPase, and phosphorylation and activation of the kinase Src. Interestingly, ouabain effects do not extend to all tyrosine kinases, since phosphorylation of the Src related, Fyn kinase, remains unchanged after ouabain treatment. This suggests kinase specificity for the action of ouabain in ADPKD cells. Similar observations were made in mouse embryonic cells functionally null in the kinases Src, Yes, and Fyn. In these cells, the lack of ouabain effect was rescued only after expression of Src, with Yes and Fyn being not required (19). The sensitivity of ouabain-dependent phosphorylation of EGFR and caveolin and the decrease in association of the EGFR with the Na-K-ATPase in response to PP2 also support a primary role of Src in the ouabain-dependent Na-K-ATPase signaling machinery of ADPKD cells. Previous work showed that ouabain causes transactivation of EGFR through Src (49). Our data agree with Src influencing EGFR phosphorylation, but the reduction in P-Src by tyrphostin AG1478 suggests that Src phosphorylation is affected by EGFR as well. It is possible that, in ADPKD cells, there is a cross talk between EGFR and Src, which could be dependent or independent on ouabain stimulation. Although more experiments are required to prove this possibility, an upstream effect of EGFR on Src will not be an event unique to ADPKD cells, since this has been reported in breast cancer cells (21). Previous work showed that the kidney cystic epithelium is susceptible to the proliferative effects of the EGF and that activation of EGFR plays an important function in ADPKD cyst formation and growth (48). In addition, a correlation between Src activity and severity of ADPKD has been reported (39). Those observations prompted researchers to use inhibitors of the EGFR and Src as possible therapies to slow progression of the disease (10, 38, 39, 41). It is possible that EGFR and Src are common intermediaries of the effect of different ligands affecting growth of ADPKD cells. Our results reveal ouabain as a compound that can act through the EGFR and Src, in a manner that is independent from EGF. Therefore, ouabain constitutes an additional factor that can activate the EGFR and Src pathway to stimulate ADPKD cell proliferation.
We found that ouabain stimulates phosphorylation and activity of B-Raf and increases P-ERK levels. Ouabain activation of B-Raf is sensitive to PP2 and tyrphostin AG1478, whereas ERK activation is prevented by those inhibitors and U0126. This suggests that as downstream events of EGFR and Src activation, ouabain-dependent Na-K-ATPase signaling impinges on the B-Raf-MEK-ERK pathway. Our results show that ouabain-induced phosphorylation of ERK is greater than the one we found for other upstream mediators. This suggests the possibility that the ouabain-triggered signal becomes amplified during the process of intracellular transduction, as previously proposed (20). The B-Raf-MEK-ERK pathway plays a key role in ADPKD, and it constitutes an essential mediator of ADPKD cell growth and cyst development (6). B-Raf has been shown to be aberrantly expressed and to exhibit cAMP-dependent activation in ADPKD cystic renal epithelial cells (33, 50). These alterations constitute a mechanism by which cAMP increases ADPKD cell growth (26, 50). Our results show that ouabain acts on the abnormal B-Raf pathway to affect ADPKD cells.
Once activated, ERK is known to translocate to the cell nucleus, where it regulates gene expression by activating a series of transcription factors (52). We found that ouabain activation of ERK phosphorylation is followed by internalization of ERK to the ADPKD cell nuclei, suggesting that this is a mechanism by which ouabain can regulate transcription of genes involved in cell proliferation. A complete characterization of the genes that are targeted by this ERK in ADPKD cells is beyond the scope of the present work. However, particularly relevant to the effects of ouabain in ADPKD cell growth is the regulation of proteins involved in the cell cycle. Thus, ouabain reduces the expression levels of two of the fundamental CKI proteins, p21 and p27. Repression of p21 and p27 has been shown to enhance epithelial cell proliferation and cyst growth in a mouse model of ADPKD (1). Therefore, ouabain regulation of p21 and p27 represents a mechanism that can influence ADPKD epithelial cell growth. The ability of cardiotonic steroids to regulate CKI proteins has also been demonstrated in human cancer cell lines. Thus, in lung cancer cells, ouabain inhibits synthesis of p53 as a consequence of activation of the Src and MAPK signaling pathway (46). Our results show that in a metaplastic disease, such as ADPKD, ouabain is also able to regulate expression of CKI proteins.
In conclusion, our results show that ouabain-induced proliferation of ADPKD cells is mediated via the plasma membrane Na-K-ATPase signalosome, including the EGFR-Src-B-Raf-MEK/ERK pathways and the downstream cyclin kinase inhibitors p21 and p27. In contrast to ADPKD cells, our previous data (27) and our present observations suggest that ouabain does not have significant effects in proliferation of normal human kidney cells. These results highlight the relevance of ouabain as a factor affecting diseased cells. Because ouabain enhances cell proliferation, a key characteristic of ADPKD cyst development, it represents an agent that, circulating in blood, can potentially influence the outcome of ADPKD in a negative manner. Supporting this is the abnormally increased affinity that ADPKD cells exhibit for ouabain (27), which makes these cells more sensitive to the effects for the hormone. Concomitantly, it is possible that endogenous ouabain levels are elevated in patients with ADPKD, which will exacerbate the initial or continuos growth of the kidney cystic cells. In this respect, determination of endogenous ouabain levels in patients with ADPKD will be important. In any case, our data here provide new evidence for the sensitivity of ADPKD cells to ouabain and provide novel information on the intracellular pathways involved in the effects of ouabain in ADPKD cell proliferation. This could be potentially used to develop therapeutic strategies to inhibit ouabain-dependent Na-K-ATPase signaling to delay ADPKD progression.
GRANTS
This work was supported by National Institutes of Health Grants DK081431 and HD043044 to G. Blanco and DK081579 to D. Wallace.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank the assistance of the PKD Foundation and the Biospecimen Shared Resource at the University of Kansas Medical Center and the hospitals participating in the Polycystic Kidney Research Retrieval Program for providing the human ADPKD kidneys from which the cells used in this study were obtained.
REFERENCES
- 1. Alcalay NI, Sharma M, Vassmer D, Chapman B, Paul B, Zhou J, Brantley JG, Wallace DP, Maser RL, Vanden Heuvel GB. Acceleration of polycystic kidney disease progression in cpk mice carrying a deletion in the homeodomain protein Cux1. Am J Physiol Renal Physiol 295: F1725–F1734, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allen JC, Abramowitz J, Koksoy A. Low concentrations of ouabain activate vascular smooth muscle cell proliferation. Ann NY Acad Sci 986: 504–508, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Aperia A. New roles for an old enzyme: Na-K-ATPase emerges as an interesting drug target. J Intern Med 261: 44–52, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Bhunia AK, Piontek K, Boletta A, Liu L, Qian F, Xu PN, Germino FJ, Germino GG. PKD1 induces p21(waf1) and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell 109: 157–168, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Blaustein MP, Juhaszova M, Golovina VA. The cellular mechanism of action of cardiotonic steroids: a new hypothesis. Clin Exp Hypertens 20: 691–703, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Calvet JP. MEK inhibition holds promise for polycystic kidney disease. J Am Soc Nephrol 17: 1498–1500, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Calvet JP, Grantham JJ. The genetics and physiology of polycystic kidney disease. Semin Nephrol 21: 107–123, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Chueh SC, Guh JH, Chen J, Lai MK, Teng CM. Dual effects of ouabain on the regulation of proliferation and apoptosis in human prostatic smooth muscle cells. J Urol 166: 347–353, 2001 [PubMed] [Google Scholar]
- 9. Dmitrieva RI, Doris PA. Ouabain is a potent promoter of growth and activator of ERK1/2 in ouabain-resistant rat renal epithelial cells. J Biol Chem 278: 28160–28166, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Elliott J, Zheleznova NN, Wilson PD. c-Src inactivation reduces renal epithelial cell-matrix adhesion, proliferation and cyst formation. Am J Physiol Cell Physiol In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feraille E, Doucet A. Sodium-potassium-adenosinetriphosphatase-dependent sodium transport in the kidney: hormonal control. Physiol Rev 81: 345–418, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Grantham JJ. Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med 359: 1477–1485, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Haas M, Wang H, Tian J, Xie Z. Src-mediated inter-receptor cross-talk between the Na+/K+-ATPase and the epidermal growth factor receptor relays the signal from ouabain to mitogen-activated protein kinases. J Biol Chem 277: 18694–18702, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Kaplan JH. Biochemistry of Na-K-ATPase. Annu Rev Biochem 71: 511–535, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Kometiani P, Li J, Gnudi L, Kahn BB, Askari A, Xie Z. Multiple signal transduction pathways link Na+/K+-ATPase to growth-related genes in cardiac myocytes. The roles of Ras and mitogen-activated protein kinases. J Biol Chem 273: 15249–15256, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Koster JC, Blanco G, Mercer RW. A cytoplasmic region of the Na-K-ATPase alpha-subunit is necessary for specific alpha/alpha association. J Biol Chem 270: 14332–14339, 1995 [DOI] [PubMed] [Google Scholar]
- 17. Li W, Sanki A, Karim RZ, Thompson JF, Soon Lee C, Zhuang L, McCarthy SW, Scolyer RA. The role of cell cycle regulatory proteins in the pathogenesis of melanoma. Pathology 38: 287–301, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Liang M, Tian J, Liu L, Pierre S, Liu J, Shapiro J, Xie ZJ. Identification of a pool of nonpumping Na/K-ATPase. J Biol Chem 282: 10585–10593, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Liu J, Kesiry R, Periyasamy SM, Malhotra D, Xie Z, Shapiro JI. Ouabain induces endocytosis of plasmalemmal Na/K-ATPase in LLC-PK1 cells by a clathrin-dependent mechanism. Kidney Int 66: 227–241, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Liu L, Askari A. On the importance and mechanism of amplification of digitalis signal through Na+/K+-ATPase. Cell Mol Biol (Noisy-le-grand) 52: 28–30, 2006 [PubMed] [Google Scholar]
- 21. Mader CC, Oser M, Magalhaes MA, Bravo-Cordero JJ, Condeelis J, Koleske AJ, Gil-Henn H. An EGFR-Src-Arg-cortactin pathway mediates functional maturation of invadopodia and breast cancer cell invasion. Cancer Res 71: 1730–1741, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marshall CB, Shankland SJ. Cell cycle and glomerular disease: a minireview. Nephron Exp Nephrol 102: e39–e48, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Marshall CB, Shankland SJ. Cell cycle regulatory proteins in podocyte health and disease. Nephron Exp Nephrol 106: e51–e59, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Mohammadi K, Liu L, Tian J, Kometiani P, Xie Z, Askari A. Positive inotropic effect of ouabain on isolated heart is accompanied by activation of signal pathways that link Na+/K+-ATPase to ERK1/2. J Cardiovasc Pharmacol 41: 609–614, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Murata Y, Matsuda T, Tamada K, Hosoi R, Asano S, Takuma K, Tanaka K, Baba A. Ouabain-induced cell proliferation in cultured rat astrocytes. Jpn J Pharmacol 72: 347–353, 1996 [DOI] [PubMed] [Google Scholar]
- 26. Nagao S, Yamaguchi T, Kusaka M, Maser RL, Takahashi H, Cowley BD, Grantham JJ. Renal activation of extracellular signal-regulated kinase in rats with autosomal-dominant polycystic kidney disease. Kidney Int 63: 427–437, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Nguyen AN, Wallace DP, Blanco G. Ouabain binds with high affinity to the Na-K-ATPase in human polycystic kidney cells and induces extracellular signal-regulated kinase activation and cell proliferation. J Am Soc Nephrol 18: 46–57, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Nishio S, Hatano M, Nagata M, Horie S, Koike T, Tokuhisa T, Mochizuki T. Pkd1 regulates immortalized proliferation of renal tubular epithelial cells through p53 induction and JNK activation. J Clin Invest 115: 910–918, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ong AC, Harris PC. Molecular pathogenesis of ADPKD: the polycystin complex gets complex. Kidney Int 67: 1234–1247, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Park JY, Schutzer WE, Lindsley JN, Bagby SP, Oyama TT, Anderson S, Weiss RH. p21 Is decreased in polycystic kidney disease and leads to increased epithelial cell cycle progression: roscovitine augments p21 levels. BMC Nephrol 8: 12, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pierre SV, Xie Z. The Na-K-ATPase receptor complex: its organization and membership. Cell Biochem Biophys 46: 303–316, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Quest AF, Leyton L, Parraga M. Caveolins, caveolae, and lipid rafts in cellular transport, signaling, and disease. Biochem Cell Biol 82: 129–144, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Schmitt JM, Stork PJ. PKA phosphorylation of Src mediates cAMP's inhibition of cell growth via Rap1. Mol Cell 9: 85–94, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Schoner W. Endogenous cardiac glycosides, a new class of steroid hormones. Eur J Biochem 269: 2440–2448, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Schoner W, Scheiner-Bobis G. Endogenous cardiac glycosides: hormones using the sodium pump as signal transducer. Semin Nephrol 25: 343–351, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Stan RV. Structure of caveolae. Biochim Biophys Acta 1746: 334–348, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Sutters M. The pathogenesis of autosomal dominant polycystic kidney disease. Nephron Exp Nephrol 103: e149–e155, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Sweeney WE, Jr, Hamahira K, Sweeney J, Garcia-Gatrell M, Frost P, Avner ED. Combination treatment of PKD utilizing dual inhibition of EGF-receptor activity and ligand bioavailability. Kidney Int 64: 1310–1319, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Sweeney WE, Jr, von Vigier RO, Frost P, Avner ED. Src inhibition ameliorates polycystic kidney disease. J Am Soc Nephrol 19: 1331–1341, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Torres VE, Harris PC. Polycystic kidney disease: genes, proteins, animal models, disease mechanisms and therapeutic opportunities. J Int Med 261: 17–31, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Torres VE, Sweeney WE, Jr, Wang X, Qian Q, Harris PC, Frost P, Avner ED. EGF receptor tyrosine kinase inhibition attenuates the development of PKD in Han:SPRD rats. Kidney Int 64: 1573–1579, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Trevisi L, Pighin I, Luciani S. Vascular endothelium as a target for endogenous ouabain: studies on the effect of ouabain on human endothelial cells. Cell Mol Biol (Noisy-le-grand) 52: 64–70, 2006 [PubMed] [Google Scholar]
- 43. Wallace DP. Cyclic AMP-mediated cyst expansion. Biochim Biophys Acta In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wallace DP, Grantham JJ, Sullivan LP. Chloride and fluid secretion by cultured human polycystic kidney cells. Kidney Int 50: 1327–1336, 1996 [DOI] [PubMed] [Google Scholar]
- 45. Wang H, Haas M, Liang M, Cai T, Tian J, Li S, Xie Z. Ouabain assembles signaling cascades through the caveolar Na+/K+-ATPase. J Biol Chem 279: 17250–17259, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Wang Z, Zheng M, Li Z, Li R, Jia L, Xiong X, Southall N, Wang S, Xia M, Austin CP, Zheng W, Xie Z, Sun Y. Cardiac glycosides inhibit p53 synthesis by a mechanism relieved by Src or MAPK inhibition. Cancer Res 69: 6556–6564, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wilson PD. Polycystic kidney disease: new understanding in the pathogenesis. Int J Biochem Cell Biol 36: 1868–1873, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Wilson SJ, Amsler K, Hyink DP, Li X, Lu W, Zhou J, Burrow CR, Wilson PD. Inhibition of HER-2(neu/ErbB2) restores normal function and structure to polycystic kidney disease (PKD) epithelia. Biochim Biophys Acta 1762: 647–655, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Xie Z, Cai T. Na+-K+-ATPase-mediated signal transduction: from protein interaction to cellular function. Mol Interv 3: 157–168, 2003 [DOI] [PubMed] [Google Scholar]
- 50. Yamaguchi T, Nagao S, Wallace DP, Belibi FA, Cowley BD, Pelling JC, Grantham JJ. Cyclic AMP activates B-Raf and ERK in cyst epithelial cells from autosomal-dominant polycystic kidneys. Kidney Int 63: 1983–1994, 2003 [DOI] [PubMed] [Google Scholar]
- 51. Yamaguchi T, Pelling JC, Ramaswamy NT, Eppler JW, Wallace DP, Nagao S, Rome LA, Sullivan LP, Grantham JJ. cAMP stimulates the in vitro proliferation of renal cyst epithelial cells by activating the extracellular signal-regulated kinase pathway. Kidney Int 57: 1460–1471, 2000 [DOI] [PubMed] [Google Scholar]
- 52. Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors 24: 21–44, 2006 [DOI] [PubMed] [Google Scholar]