Figure 6.
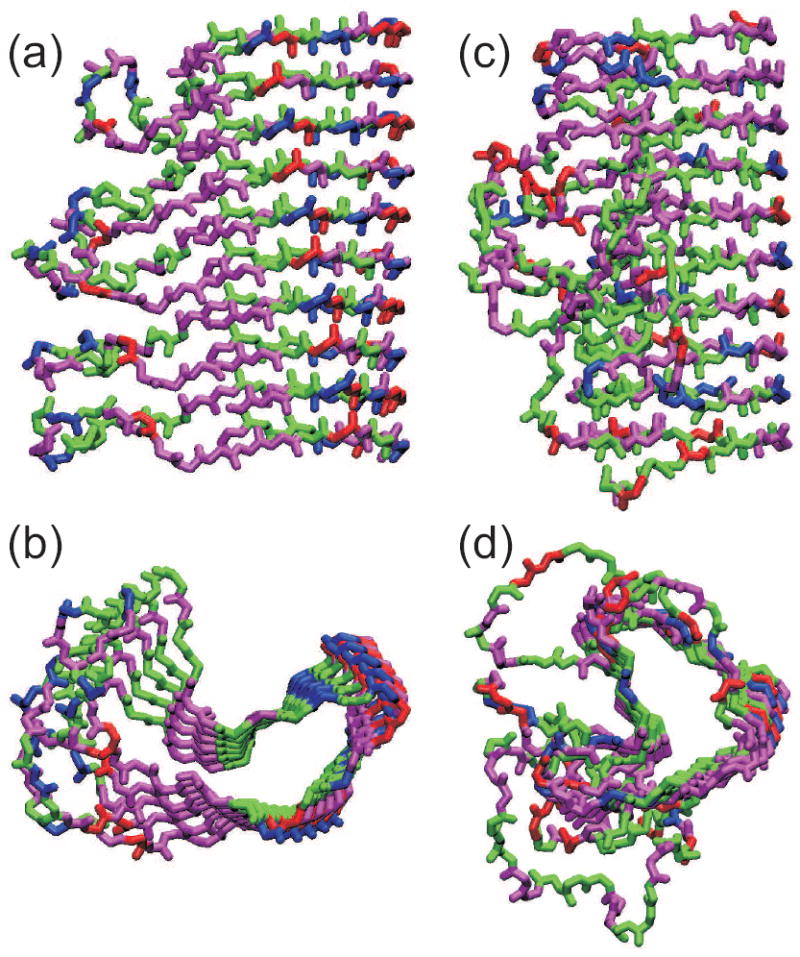
Comparison of the HET-s218-289 prion fibril structure (a,b) with that of the β-helical protein Bordetella pertussis pertactin (c,d), based on Protein Data Bank files 2RNM and 1DAB, respectively. Structures are viewed perpendicular (a,c) and parallel (b,d) to the long axis of the fibril or β-helix. For HET-s218-289, five repeats of residues 226-278 are shown, with each repeat forming two “rungs” of the β-helix. For pertactin, residues 1-285 is shown. Hydrophobic, negatively charged, postively charged (including His), and polar (including Gly) residues are colored green, red, blue, and magenta, respectively.
