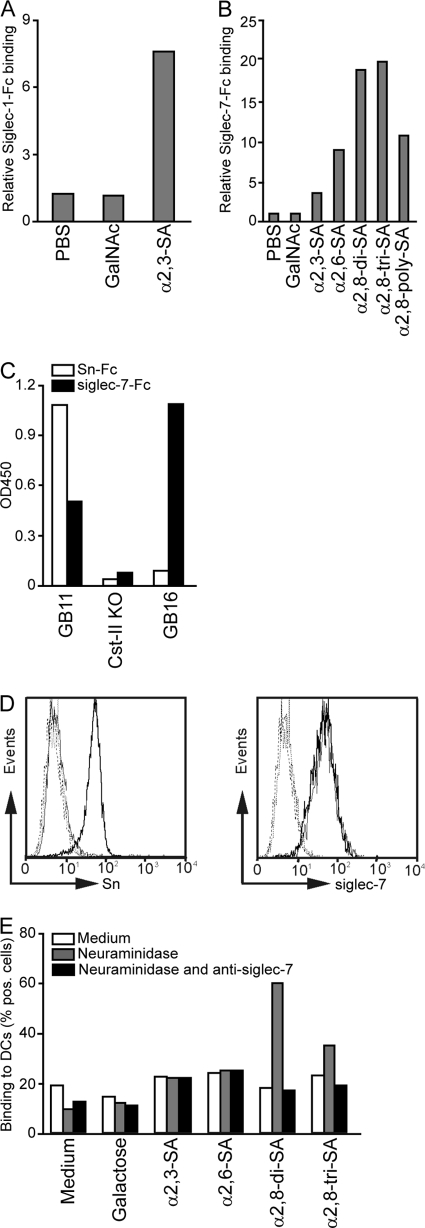
Fig. 2.
Sn and siglec-7 have different binding specificities toward sialylated LOS structures. (A and B) Sn-Fc (A) and siglec-7-Fc (B) proteins were precomplexed with anti-human-Fc PO and added to coated PAA-conjugated glycan structures. As a control for the sialic acid specificity of the siglec-Fc proteins, uncoated wells or GalNAc-PAA-coated wells were used. Depicted are the relative bindings of precomplexed siglec-Fc proteins compared to the binding of secondary antibody alone. (C) Sn-Fc and siglec-7-Fc precomplexed with anti-human-Fc PO binding to coated GB11 (α2,3-linked sialic acid), the Cst-II mutant (without sialic acid), and GB16 (α2,8-linked sialic acid) LOS. (D) Fluorescence-activated cell sorter (FACS) analysis of immature DCs (thin lines) and mature DCs (bold lines) stained with MAb 7D2 (anti-Sn, left) or MAb S7.5A (anti-siglec-7, right). Dotted lines are for the corresponding negative controls. (E) Binding of biotinylated glycosylated PAA-conjugated probes to immature DCs as measured by flow cytometry. Binding to nontreated DCs (medium), neuraminidase-pretreated DCs (gray bars), or neuraminidase- and anti-siglec-7-pretreated DCs (black bars) of one representative donor is depicted. The percentage of cells binding the PAA-glycoconjugates is depicted. For all experiments, results from one out of five representative experiments are shown.