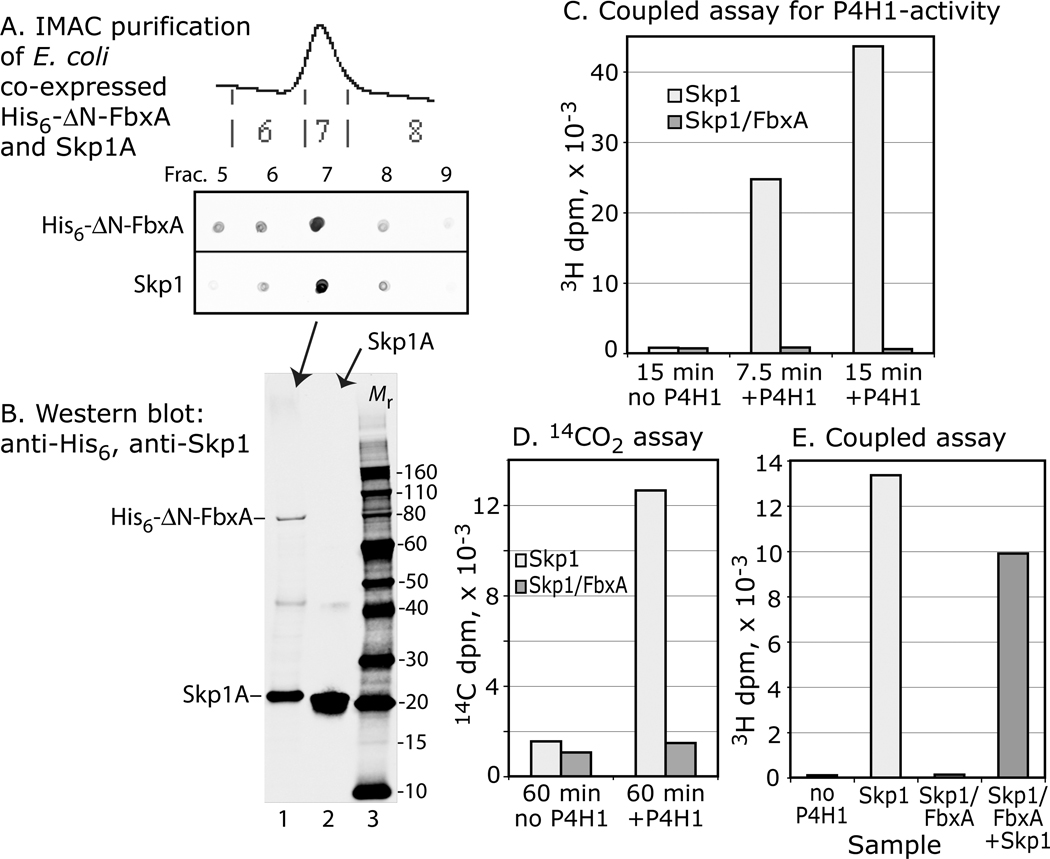
Figure 7. Skp1 complexed with FbxA is not a substrate. Skp1A and His6-⊗N-FbxA were co-expressed in E. coli and purified by Ni+2-IMAC.
(A) Dot-blot analyse s show that Skp1A copurified with His6-⊗N-FbxA. (B) Western blot analysis confirmed the presence of His6-⊗N-FbxA and Skp1A in frac. 7 (lane 1), and Skp1A in a Skp1A preparation (lane 2); blots were used to estimate relative levels of Skp1A for the assays. (C) Coupled, 1-step 3H-assay showing absence of acceptor activity for the purified Skp1A/FbxA complex, in comparison with a similar level of free Skp1A in a parallel reaction. (D) Similar analysis using the 14C-release assay. (E) Addition of an equal amount of free Skp1A to the reaction with Skp1A/His6-⊗N-FbxA resulted in near normal acceptor activity. Data are representative of two independent experiments.