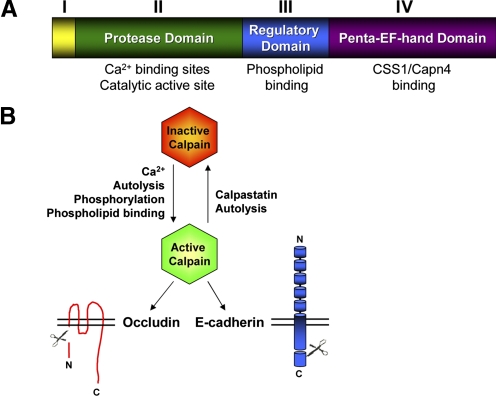
Figure 2.
Calpains cleave occludin and E-cadherin. (A) A schematic representation of the 80-kD large subunit of the classical calpains is shown. The N-terminal Domain I undergoes intermolecular autolysis upon calpain activation. Ca2+-binding sites and catalytic activity are contained within Domain II. Domain III comprises a C2-like domain that contains sites for phospholipid binding. Five consecutive EF-hand motifs make up Domain IV and contribute to Ca2+ binding of the large subunits and dimerization with the small regulatory subunit, CSS1/Capn4. (B) Calpains are positively regulated by Ca2+-binding, Domain I autolysis, phosphorylation, and phospholipid binding and inhibited by calpastatin and autolysis. The N-terminus (N) of occludin and the C-terminus (C) of E-cadherin are targets for calpain cleavage.