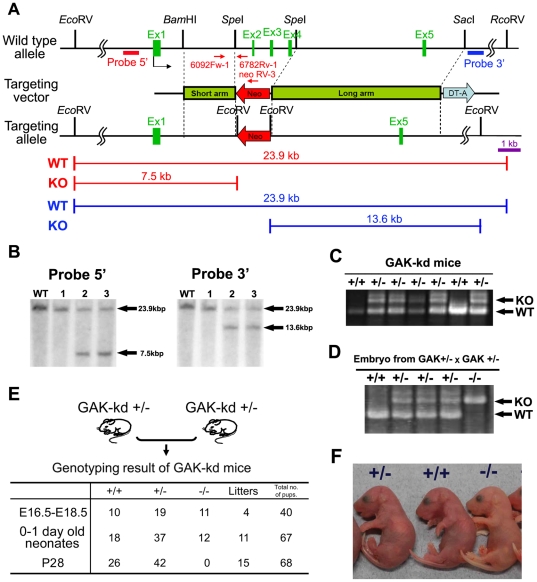
Figure 1. Generation of GAK kinase dead mice and establishment of MEFs.
(A) Schematic representation of the wild-type mouse GAK locus (top), targeting vector (middle), and targeted locus (bottom). The coding exons are indicated as black boxes. To delete the kinase domain of GAK, the coding exons 2 (Ex2), 3 (Ex3), and 4 (Ex4) were replaced with a neomycin selection cassette (Neo). The diphtheria toxin A gene (DT-A) was used for negative selection. The arrows indicate the position and orientation of PCR primers for genotyping. The position of Southern blotting probes (probe 5′ and 3′) are shown as red and blue boxes on the first line, respectively. The restriction fragments of wild-type (WT) and targeted kinase (KO) are shown below. (B) Southern blot analysis of genomic DNA from ES clones. Homologous recombination of the targeting vector into the GAK locus generates two additional EcoRV sites. Genomic DNA was digested with EcoRV and hybridized with 5′ (left panel) and 3′ (right panel) probes. Wild type (23.9 kb) and mutated fragments (7.5 and 13.6 kb) are indicated. WT lane is a negative control. (C) PCR analysis of genomic DNA from the tails of GAK-kd adult mice. Amplification products corresponding to WT and mutated (KO, knockout) alleles (691 and 997 bases, respectively) are shown. (D) PCR analysis of genomic DNA of embryos obtained from heterozygote intercrosses. (E) Genotypic ratio of embryos (embryonic days 16.5–18.5), newborns (containing pups delivered by Cesarean section), and weanlings (postnatal days 28) obtained from heterozygote intercrosses. (F) Gross morphology of newborns obtained from heterozygote intercrosses.