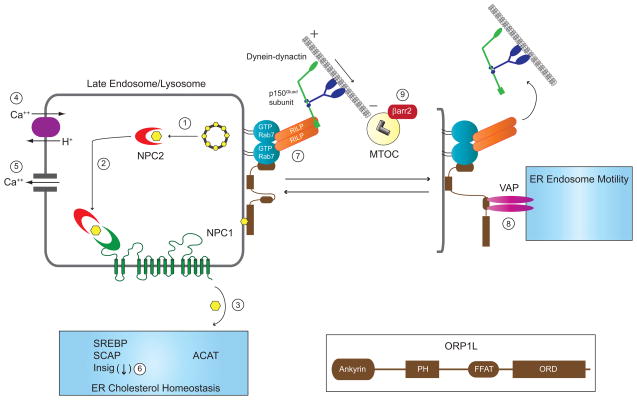
Figure 3.
NPC and CF converge on several mechanisms involved in LE/Ly function, ER cholesterol homeostasis and quality control, and MT-dependent organelle motility. In the prevailing model for NPC protein function, soluble NPC2 mobilizes free cholesterol from LBPA-rich internal membranes in multivesicular LEs (1). NPC2 then delivers free cholesterol to the N-terminal loop of NPC1 (2), and free cholesterol is exported from LE/Ly to other cellular membranes including the ER by an unknown mechanism (3). An alternative model proposes that NPC1 controls proton-dependent LE/Ly calcium filling via an unknown mechanism (4). LE/Ly calcium fuels local elevations in cytosolic calcium required for LE/Ly fusion (5). The ER houses multiple sterol-sensing proteins involved in cholesterol homeostasis. Stringent ER quality control has an important role in cholesterol homeostasis as several ER stressors bypass cholesterol inhibition of SREBP trafficking to the Golgi by depleting Insig-1 (6). LE/Ly motility and positioning is regulated by GTP-Rab7 effectors RILP and ORP1L that reversibly couple these organelles to minus end directed dynein-dynactin MT motors. ORP1L has several protein interaction modules including amino-terminal ankyrin repeats that bind GTP-Rab7, a pleckstrin homology (PH) domain, a FFAT motif capable of binding VAP located on ER membranes, and an oxysterol regulatory domain (ORD) (see inset). When the ORP1L ORD is occupied by LE cholesterol, the Rab7-RILP-ORP1L complex assumes a conformation allowing it to bind minus end directed dynein-dynactin MT motors (7). The ORP1L FFAT motif is exposed under low LE/Ly sterol conditions facilitating interactions with VAP that trigger release of dynein-dynactin MT motor complexes from RILP (8). Unopposed dynein-dynactin MT activity as in NPC impairs LE/Ly motility causing accumulation of cholesterol-filled LSOs at the MTOC. CF conceivably impairs LE/Ly motility triggering a similar response by interfering with normal function of βarr2 at the MTOC (9).