Abstract
During the last decade considerable attention has been focussed upon the development of new technologies and methodologies for detection of drug resistance in Mycobacterium tuberculosis. There is a growing acknowledgement that the redundancy in testing a full panel of first-line drugs is an unaffordable indulgence; since only resistance at baseline to either (or both) of the two most potent agents, isoniazid (H) and rifampicin (R), would usually prompt therapeutic modification there is a shift towards initial RH (or R alone for selected genotypic technologies) drug susceptibility testing (DST) followed, if necessary by further extended first and second line agent (currently phenotypic) DST. Most of the new drug susceptibility tests endorsed by the World Health Organization since 2007 deliver rapid RH (or R alone for selected genotypic technologies) DST. Targeting of patient groups with risk factors for drug resistance increases the proportion of tests that identify drug resistance, but in many settings at least as many patients with drug resistant disease will have no identifiable risk factors—equity of care demands that universal RH DST at baseline should be the goal. We review the bewildering array of choices facing TB program directors and attempt to provide objective information to help in deciding what tools may be best suited to different environments.
ACTIONABLE INFORMATION
For the past couple of decades, during which the standard tuberculosis (TB) regimen has included ≥3 of rifampicin (R), isoniazid (H), ethambutol (E), and pyrazinamide (Z), the conventional tuberculosis (TB) drug-susceptibility test (DST) panel has comprised R, H, E, and streptomycin (S). Results are rarely available before patients step down to maintenance RH therapy after 2 months. Therefore, the E and S data are almost never of any consequence unless resistance to either or both of the 2 most potent agents, R and H, is identified. However, for a test that takes 3–4 months from the time that the sputum sample is taken to the result being returned to the physician, in well-resourced settings, it has been regarded as pragmatic (and not much additional work) to add in a couple of additional tubes (for E and S), so that such information, if ever needed, is available immediately, rather than another 6–8 weeks later. That these largely unhelpful data are generated in laboratories across the globe, whereas DST for Z, the third most potent agent after R and H, has been so neglected because of technical challenges is inexplicable. “Never request a test without having a plan for the result” has long been a favored refrain of mentors to junior physicians. However, we are failing to perform tests that might make a difference (Z) while squandering time, resources, and incubator space on tests with little likelihood of influencing clinical decision-making. The luxury of redundant testing should surely be consigned to history, and DST strategies should move immediately toward optimizing the actionable information yield.
DISRUPTING THE CONVENTIONAL DRUG-SUSCEPTIBILITY TESTING PARADIGM
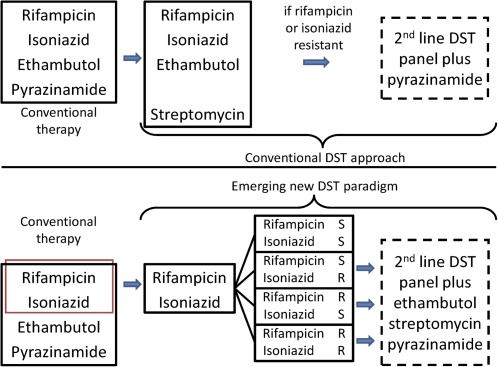
In practice in this era of rapid DST, the actionable information yield is maximized through initial R and H DST to establish whether the patient (1) has multidrug-resistant (MDR) TB and, thus, requires a second-line regimen or (2) has R or (more frequently) H monoresistance and, thus, requires modification of their consolidation phase therapy (4RH) to avoid monotherapy. Both circumstances should also trigger phenotypic DST to the remaining first-line (E, Z, and S) and second-line panels (Figure 1).
Figure 1.
Conventional and emerging approaches to tuberculosis (TB) drug-susceptibility testing (DST).
Thus, RH DST becomes the gateway to more extended testing, which is only performed when the information is needed (if the isolate is RH susceptible, it is not necessary or useful to know whether it is resistant to E or S because this will not trigger a therapeutic modification). The increasing array of phenotypic and genotypic, commercial and noncommercial rapid diagnostic tools that are now available (described below) facilitate the shift to this more streamlined approach through the delivery of reliable RH DST in a clinically useful timeframe. The challenge now becomes how to wield these tools to optimize the return; specifically, which patients should undergo RH DST and at what stage. Because of the relatively low cost and demonstrated successful pilot implementation of many of these tools, it is difficult to understand the logic of any strategy that does not entail universal RH testing at baseline.
UNIVERSAL BASELINE H AND R DST AND THE FALLACY OF TARGETED DST
In settings where resources were deemed insufficient to permit universal DST, the traditional approach required patients to experience failure of first-line TB therapy before DST would be undertaken [1]. The emergence of MDR-TB led to a risk-based assessment, so that early DST could be targeted at those with risk factors, with the remainder still being required to fail therapy before DST would be performed [2]. Failing therapy usually means persistent smear positivity after 4 months of treatment; shamefully, it also means continuing exposure of health care workers, other patients attending the health care facility, and household contacts and that patients become sicker and harder to treat with more advanced disease [3]. In the 2011 global village, this is not only appalling medicine, it is an abuse of human rights.
Even if the threshold for treatment failure is lowered, does the targeting of patients at high risk of MDR-TB make sense logistically, economically, and morally? The odds of MDR-TB in a TB-affected household contact of an index patient with MDR-TB are many times higher than those in an unexposed patient with TB; odds ratios indicate the exposures most strongly associated with the outcome (in this instance MDR-TB) and, thus, who is at greatest risk. Furthermore, they indicate that if 100 TB-affected household contacts of patients with MDR-TB are tested, many more MDR-TB cases will be detected than by testing patients with TB without an MDR-TB contact. However, even when all patients with TB with all the known MDR-TB risk factors are added, they often explain <50% of the MDR-TB detected when all TB patients are tested (D. A. J. Moore, M. H. Roper, L. Martin, unpublished data); the population attributable risk is insufficiently high to justify restricting targeted DST to these groups. The incremental cost of testing all other TB patients without identifiable risk factors for MDR is of course less rewarding; more patients have to be tested to detect each MDR-TB case. However, the alternative is to face the question, “Who am I prepared to risk not testing?” and not to hide behind the question, “Who should I test?”
The raw cost of treating MDR-TB renders any approach that reduces transmission and generation of future cases attractive; it is hard to argue in economic terms for anything other than universal RH DST at baseline rather than awaiting treatment failure. Even if the costs of treating an MDR-TB patient were as low as $10 000 [4] (which they rarely are) and testing costs were as high as $50 per patient (which they do not need to be), and one made the conservative estimate that diagnosis 4–6 months earlier would prevent only a single secondary case, one would only have to detect 1 patient with MDR-TB of 200 tested at commencement of therapy for cost savings. It is vital that there is an acknowledgment of the uncomfortable truth that we simply cannot predict MDR-TB or RH monoresistance on clinicoepidemiological grounds well enough (D. A. J. Moore, unpublished observation) and that the cost of this failure is unacceptably high, so that widespread RH testing capacity is scaled-up.
DINING FROM THE ALTERNATIVE MENU
There has been renewed enthusiasm for rapid diagnostics for drug-resistant TB over the past 2 decades, in part because of the increased global awareness and alarm raised by MDR-TB outbreaks during the 1990s [5] and more recent emergence of highly lethal strains of extensively drug-resistant (XDR) TB worldwide [6–8]. Coupled with global data about the increasing prevalence of primary (transmitted) MDR- and XDR-TB [9], there is a clear and urgent need for earlier detection of drug resistance, both to improve patient care and to interrupt transmission through effective infection control.
The classic phenotypic DST methods are based on detecting uninhibited growth of Mycobacterium tuberculosis in the presence of anti-TB drugs. Phenotypic methods are frequently used as the reference gold standard when evaluating new rapid diagnostics for MDR-TB and detect resistance regardless of the underlying molecular mechanism or resistance-conferring mutation. Until recently, DST was performed only on cultured isolates (secondary or indirect DST); however, newer methods now permit more rapid testing directly on concentrated sputum specimens. The conventional phenotypic DST methods (all indirect) include the 1% proportion method, absolute concentration, and resistance ratio, all on solid media, and the Bactec mycobacterial growth indicator tube (MGIT) system (and other commercial platforms, such as MBBacT) in liquid media [10]. Liquid culture methods are faster but at the cost of higher contamination rates and the need to confirm M. tuberculosis species with an additional biochemical or molecular test [11]. The MGIT system essentially cut by half the time for culture and DST results from 6–12 weeks to 3–4 weeks (Table 1). In 2007, the World Health Organization introduced policy guidance supporting use of liquid media for culture and DST in low- and middle-income countries [11].
Table 1.
Alternative Methods for Diagnosing Drug Resistance Endorsed by World Health Organization
| Method | Drug resistance tested | Turnaround time (range) | Cost per test (US dollar) | Pros | Cons | Reference |
| Phenotypic methods | ||||||
| Mycobacterial growth indicator tube (MGIT) | Any | 7–14 days after primary isolation (median 12–14 days) | $32–56a | Fully automated Rapid results High correlation with conventional 1% proportion method |
Need for species identification High contamination rate Cost |
11,35 |
| Nitrate reductase assay (NRA) | Isoniazid and Rifampin | 7–28 days | $3.00–4.00b | Standardized methods and protocols Less staff training than conventional DST Uses minimal equipment and readily available consumables Non-commercial, low cost |
Moderate indeterminate rate Cannot detect nitrate reductase negative M.tb (rare) |
20,28 |
| Microscopic observation drug susceptibility (MODS) | Isoniazid and Rifampin | 4–21 days | $1.40–3.50c | Standardized methods and protocols Uses minimal equipment and readily available consumables Performs well in smear-positive and smear-negative samples Non-commercial, low cost |
Requires staff training (2 weeks) to accurately identify M.tb cording Requires inverted light microscope |
13,28 |
| Colorimetric redox indicators (CRI) | Isoniazid and Rifampin | 7 days after primary isolation (delay depends upon primary culture method used) | Standardized methods and protocols Uses minimal equipment and readily available consumables Non-commercial, low cost |
Data support use as an indirect method, thus requires M.tb isolation from primary culture | 19 | |
| Genotypic methods | ||||||
| INNO-LiPA Rif | Rifampin (rpoB mutations) | 6–48 hours | $15–45 | Commercial, automated High sensitivity and specificity in smear-positive | Requires skilled, highly trained personnel (with basic molecular biology expertise) Lower sensitivity and specificity in smear-negative Does not test for INH resistance Cost |
14 |
| GenoType MTBDR and MTBDR-Plus | Rifampin and Isoniazid (rpoB and katG mutations; also inhA in MTBDR-Plus version) | 6–48 hours | $15–45 | Relatively simple High sensitivity and specificity in smear-positive High sensitivity and specificity for RIF resistance High specificity for INH resistance Validated in high-volume TB lab settings Improved sensitivity for INH resistance detection with MTBDR-Plus test |
Requires skilled, highly trained personnel (with basic molecular biology expertise) Lower performance in smear-negative Lower sensitivity for INH resistance Cost |
14,28,30–32 |
| XPert MTB/RIF | Rifampin | 1.5–2 hours | $15–120d | Commercial, automated Simple Closed system with minimal biosafety requirements |
Requires stable, uninterrupted electrical power Lower sensitivity and specificity in smear-negative Does not test for INH resistance Cost |
33,37,38 |
All costs are approximates. MGIT cost includes the following inputs: specimen transport, decontamination, test, overhead, building, equipment, staff, supplies, and primary isolation.
Only includes cost of reagents and supplies.
Only includes cost of reagents and supplies (estimated cost of inverted microscope: $1000) (13, 21).
Cost of cartridge only; varies by country. Prices expected to drop annually as global volume increases.
Because indirect methods depend on primary culture to retrieve an isolate for inoculation, DST results may take an additional 2–6 weeks. This adds up quickly to a 1–3 month delay in identification of drug resistance in patients who may suffer clinical deterioration during suboptimal therapy and who may continue to transmit drug-resistant disease to health care workers and community and family members. The alternative methods for diagnosing drug resistance thus seek to provide results more rapidly, using either direct phenotypic methods for more rapid growth and detection or genotypic methods that rely on detection of mutations in known resistance-conferring regions.
Table 1 reviews the characteristics of alternative methods for diagnosing drug resistance. There have been a number of excellent systematic reviews and meta-analyses in recent years that compare performance characteristics of these tests, which are summarized elsewhere [15, 17, 25, 26, 27].
Despite early promise during trials, phage-based assays for rapid R DST performed poorly during operational rollout, serving as an important wake-up call for diagnostic test developers. Newer versions under development include luciferase reporter phages that display a color or luminesce when TB is present [28, 29]. The nitrate reductase assay, thin layer agar assay, and colorimetric redox indicator methods have recently been reviewed and offer an attractive, low-cost, simple option for rapid detection of TB and drug resistance [15, 16, 13].
In a 2006 report of the microscopic observation drug-susceptibility (MODS) assay in Peru, results for H, R, and MDR-TB were available in a median of 7 days with 100%, 97%, and 99% agreement, respectively, compared with the reference standard. The low cost, low contamination rate, modest training requirements, and noncommercial design [24] prompted numerous additional studies showing excellent performance for both sputum and nonsputum body fluid samples from diverse settings worldwide [30–35].
On the basis of recommendations of a 2009 expert panel that systematically evaluated these phenotypic methods [15], in 2010, the World Health Organization endorsed the nitrate reductase assay and MODS and the (indirect) colorimetric redox indicator for MDR-TB DST. There was insufficient evidence to support policy guidance for use of the thin layer agar assay or phage-based assays.
With advances in our understanding of the mechanisms for TB drug resistance and with the sequencing of the M. tuberculosis genome, the field of molecular diagnostics continues to improve and offers great promise for drug resistance diagnosis. The first 2 commercially available tests—INNO-LiPA.RifTB and GenoTypeMTBDR—require conventional polymerase chain reaction (PCR) amplification, followed by hybridization onto oligonucleotide probes to detect R resistance. Both have been validated for indirect testing and on direct sputum samples with good results [17, 14, 18–20, 36]. The newer GenoTypeMTBDRPlus additionally detects isoniazid resistance-conferring mutations in inhA and katG. Recent validation studies in small-scale and high-volume laboratories have shown excellent results on smear-positive, and when used on multiple specimens, in smear-negative patients [21, 37]. Although most R resistance is mediated through mutations in the rpoB gene, making this an efficient target, a significant proportion of phenotypic H resistance is neither inhA nor katG mediated (up to 20%)[12]; thus, detection of mutations in these genes reliably indicates resistance, but the absence of a mutation does not necessarily indicate susceptibility.
The newest technology to emerge is the Cepheid XPert MTB/RIF assay, which was recently tested for direct R DST in 1730 patients from 4 countries [38]. The assay had 98% sensitivity for identification of R resistance among smear-positive patients and 74% sensitivity among smear-negative patients on the basis of testing of a single sputum specimen; specificity was 99%. Sensitivity among smear-negative patients improved with testing of a second and third specimen (up to 90%). Turnaround time was 2 hours, and training required was minimal [22, 23].
COMMERCIAL VERSUS NONCOMMERCIAL METHODS—WHICH TEST IS RIGHT FOR ME?
The cost and complexity of the aforementioned commercial tests have been a major barrier to implementation at the country level in resource-limited settings. Despite major efforts by the Foundation for Innovative New Diagnostics to negotiate concessionary prices for equipment and reagents for laboratories in high-burden settings and cost-effectiveness analyses suggesting that implementation of rapid diagnostics could provide substantial cost savings to TB programs [39–41], the initial capital investment may still be beyond reach for most countries.
A number of in-house noncommercial tests have been developed; the primary advantage is lower cost than commercial tests because of their nonproprietary nature. A significant advantage of commercial tests is the locked-down nature of the methodology, which contrasts with the standard operating procedures for noncommercial tests. Although the latter will usually have been developed and refined through numerous iterations, they are nevertheless more open to modification by the end-user, which can compromise performance. For any test, commercial or noncommercial, if the standard operating procedure is not followed and the test is not performed as it should be, the output may not be reliable; it is argued that this is less likely with a commercial test kit.
Because of the increasing array of rapid diagnostics available on the market or as in-house options, National TB Programmes (NTPs) are faced with the practical question of whether to invest in the adoption of today’s best test, with its acknowledged limitations, or to wait for perhaps a better test to come down the pipeline. The shared goal of the international community and NTPs is to provide improved diagnosis of drug resistance as close to the patient as possible, ideally with a simple point-of-care test. Although current tests are not at that stage, a number are undergoing refinements to make this a real possibility; whether this can then be translated into improved patient care with more rapid (but careful) initiation of second-line regimens in the peripheral reaches of the health care system remains to be seen.
Because of the enormous investment of time, training, infrastructure, and funds in preparing for implementation of even one test, NTPs might understandably be bewildered by choosing the direction in which to move (molecular and/or phenotypic, laboratories at what level of healthcare system, centralized or decentralized, etc.) However, the reality is that developing the laboratory infrastructure required to implement any one of the currently available tests is an investment in being able to more readily implement the next one that comes down the pipeline. Even point-of-care test implementation would still require laboratory capacity for quality assurance and extended first-line and second-line agent DST. From a human rights perspective, there is no time to wait around for the next best thing. We know from mathematical modeling that improving access to even the most basic of diagnostic tests (ie, smear microscopy) could save hundreds of thousands of lives in the next year alone [42]. Stated simply, we have waited too long already and have neglected the basic issue of laboratory and health systems strengthening for delivery of basic let alone more complex diagnostic tests.
In settings where the HIV epidemic is colliding with MDR- and XDR-TB, delays in diagnosis of TB and drug resistance are essentially a death sentence. A recent study from KwaZulu-Natal, South Africa, which has among the highest rates of MDR- and XDR-TB in the world, demonstrated that 40%–50% of patients die within 30 days after sputum sample collection [43], well before DST results are available using the indirect proportion method. For settings with a high prevalence of HIV infection, it is clear that laboratory systems must be strengthened [44].
MONITORING A DECENTRALIZED LAB SYSTEM—ARE WE CREATING SOLUTIONS OR MORE PROBLEMS?
Any program for improving delivery of laboratory services must include a solid, well-supported, quality assurance (QA) plan. As TB laboratory services become more decentralized, moving closer to the patients that most need them, the need for QA programs is even greater. Quality management is a multifaceted process that ensures adherence to standard operating procedures to provide accurate test results. Without quality standards, the reliability of an MDR-TB and XDR-TB diagnosis is questionable. However, there remains a lack of consensus at the international level about the logistics of a QA plan: how this should be done, with what frequency, and by whom. Certainly, it should encompass sample quality, assay performance (internal quality controls, reproducibility, sensitivity, specificity, rate of indeterminate results), and result delivery. The QA of commercial methods is sometimes described as superior to that of noncommercial methods, because the gathered component materials are checked for quality before dispatch. However, an excellent kit that is incorrectly used will still deliver poor results; therefore, the ultimate arbiters of quality should be further downstream. Both drug-susceptible and drug-resistant internal quality controls should be used, and a program of external quality assurance should include panel proficiency testing and/or (for direct methods for which panels of sputum samples are not available) rechecking of a proportion of results. Adequate attention to QA is a major gap in the planning (both logistic and financial) of implementation of new diagnostics and laboratory strengthening, and over-reliance on machines or kits for this is misguided. In 2010, the New Diagnostics Working Group of the STOP-TB Partnership completed online publication (at www.tbevidence.org) of all up-to-date standard operating procedures for culture-based DST methods with all available QA guidance materials for each method in a step toward improving access to this information.
The major barriers to providing quality diagnosis and implementing a QA plan involve broader issues related to the overwhelming shortage of well-trained laboratory staff, underemphasis or complete absence of laboratory strengthening in national budgets and strategic plans, and weak technical and physical health care infrastructure for delivery of specimens and reporting of results. Thus, a critical first step to improving quality diagnostic services is budgeting for and strengthening of both human and laboratory resources. Quality management issues are a challenge for many national reference laboratories but will be even more complex when considering decentralized laboratory systems where issues of trained staff and logistics are greater. As rapid diagnostic tests move to the point of care, greater attention to QA plans is needed to ensure optimal performance and impact.
TRANSFORMING VICIOUS INTO VIRTUOUS CIRCLES
Only 7% of the total MDR-TB cases estimated worldwide were accurately diagnosed in 2008 [45], and of these, <20% received appropriate treatment for MDR-TB. The cost and complexity of MDR- and XDR-TB treatment are substantially greater than that of drug-susceptible TB and present a formidable challenge to already constrained budgets and personnel of most NTPs. The 2009 guidelines for TB drug resistance surveillance, together with the World Health Assembly and the International Standards for TB Care, call for universal access to diagnosis and treatment of MDR-TB. Specifically, the guidelines state that persons who receive a diagnosis of MDR-TB through drug resistance surveys should be treated [10]. However, an ethical dilemma arises as countries seek to improve MDR-TB diagnosis and surveillance with whichever diagnostic modality they choose while still lacking the capacity to provide treatment for all identified cases. Although mechanisms, such as the Green Light Committee, assist countries in providing MDR-TB treatment, a critical first step is determining the magnitude of the MDR-TB burden in a country or region.
How does an NTP break out of this vicious cycle of not testing for MDR-TB because of lack of treatment, but not having MDR-TB treatment because of lack of accurate case estimates? Is it ethical to improve MDR-TB diagnostic services without a concomitant plan for increasing MDR-TB treatment? What are the practical, human, and financial resources needed to address this, and whose responsibility is it? This debate echoes that of HIV testing scale-up and antiretroviral treatment. One can similarly reason that knowledge of a patient’s TB drug resistance status empowers patients, health care providers, and policy makers to develop an action plan for adequate treatment and prevention. Remaining in the dark about the magnitude of the MDR- and XDR-TB epidemic serves no identifiable purpose, and knowledge can clearly provide leverage and direction for control efforts. Indeed, diagnosis and disease surveillance are the first step in controlling any infectious disease epidemic, as noted by the father of TB, Robert Koch, in his 1905 Nobel Lecture: “The starting point in the fight against all contagious diseases is the obligation to report, because without this most cases of the disease remain unknown” [42].
Case study
Box 1. Since 2005, Tugela Ferry, South Africa, has been one of the only settings with a high prevalence of TB and HIV infection worldwide that has been conducting continuous drug resistance surveillance for all patients with suspected TB [46]. Although MDR-TB treatment capacity lagged initially, resulting in long waiting lists of up to 2 months for some patients, accurate case estimates resulted in development of an emergency response plan, mobilization of resources, rapid expansion of inpatient MDR-TB treatment capacity, and creation of novel community-based treatment programs for MDR-TB [47, 48]. In addition, although patients with MDR-TB were awaiting treatment initiation, infection control measures were immediately implemented, such as patient isolation and household contact tracing. Together, these efforts may be beginning to turn the tide on the MDR-TB epidemic in Tugela Ferry, with the 2009 caseload decreased to 33% (n = 82) from a 2006 peak (n = 123; C. Marra, personal communication). Improved diagnosis and surveillance should be viewed as the catalyst toward achieving universal access to MDR-TB treatment and breaking the truly vicious cycle of diagnostic delay, clinical decline, ongoing transmission, and increasing MDR-TB incidence.
HORSES FOR COURSES—WHAT METHOD IS BEST SUITED TO MY CONTEXT?
Despite recent market and media attention to improving MDR-TB diagnostics, it is surprising and disappointing that the likelihood that a poor rural Sri Lankan farmer or Bolivian taxi driver will ever get access to a TB DST has not improved. The vast under-delivery of MDR-TB diagnostics when so much money is being spent on them is bewildering. The reality is that there is no single true replacement technology that serves all levels of the health care system. Calls for a global diagnostic laboratory to which all TB DNA could be sent for genotypic DST ignores (among other things) the reality that simply getting a sputum sample 3 miles across a Latin American city from a microscopy center to a regional reference laboratory in a fair condition is a major logistical challenge.
Budgetary constraints are a major consideration, but how to address them is not immediately obvious; the choice between 5 $10 tests or 1 equally reliable $50 test might appear straightforward. However, if the $50 test provides the result for 1 patient at particular high risk in 1 day, this might be more useful than having results for 5 persons 1 week later. The existing environment to which new MDR-TB testing capacity is introduced may be an important determinant of which test to choose. Automated phenotypic platforms, such as the MGIT960, facilitate high throughput that few manual systems can match and are therefore well suited to busy regional laboratories but are clearly less appropriate for a district laboratory receiving 30 samples per week for DST. However, the sum of the demand from all the district laboratories wishing to run 30 DST samples per week is enormous and unmet. The direct nitrate reductase assay on solid media uses a modified Lowenstein–Jensen media and should thus be straightforward for laboratories already performing Lowenstein–Jensen cultures, although the need for repeated opening of cultures demands category III level laboratory biosafety. The MODS assay offers the advantages of being a low-cost liquid culture TB diagnostic and supporting direct RH DST on acid-fast bacilli smear-negative samples. Moreover, biosafety is good because cultures are never opened, thus, it can be performed in a category II facility.
HOW MANY PATIENTS COULD I PROVIDE WITH H AND R DST WITH A CONSUMABLES BUDGET OF $250 000?
In this exercise, we explore 4 ways in which $250 000 could be spent on MDR-TB detection with use of each of the 6 tools shown (Figure 2; Table 2). No consideration is given to the downstream costs or savings from early diagnosis of MDR-TB, including those associated with treatment or further laboratory testing, such as second-line DST. Performance of the methodologies for the indications for which they have been validated are assumed to be essentially indistinguishable; thus, this exercise is simply about value available for a fixed budget. MODS and nitrate reductase assay permit testing of the highest number of patients, and the advantage of direct tests over indirect tests requiring primary isolation is evident.
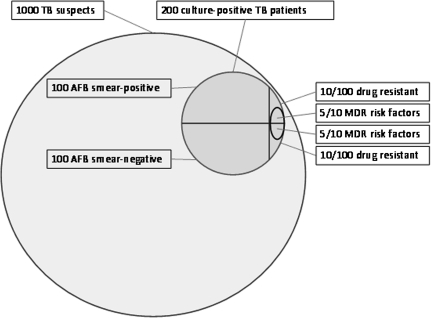
Figure 2.
Epidemiological scenario for crude costing exercise. Two hundred of 1000 patients with suspected tuberculosis (TB) have active (culture-positive) TB; 40 (20%) report a recognized risk factor for multidrug-resistant (MDR) TB [50]; 100 are smear positive and 100 are smear negative; 20 (10%) have drug-resistant TB (10 smear-positive, 10 smear-negative). Ten patients (50%; D. Moore, unpublished data) with drug-resistant TB have an identifiable risk factor (5 smear-positive, 5 smear-negative).
Table 2.
Number of Individuals That Could Undergo RH DST (R alone for Xpert MTB/RIF) Under Four Different Testing Strategies Utilizing Each of the Six Rapid DST Tools Within a Consumables Budget of $250 000
| All TBsuspects | All smear-positives | All MDR high-risk TB patients | All MDR high-risk smear-positives | |
| Testing approach | Could test this many unselected TB suspects a | Could test this many smear-positive patients | Could test this many MDR high-risk patients (and still fail to detect half of all MDR patients) | Could test this many MDR high-risk smear-positives (and still fail to detect half of smear+ MDR patients) |
| LJ culture and proportion method DST b | 60 650 | 21 834 | 60 650 | 21 834 |
| MGIT culture and DST b | 31 125 | 12 450 | 31 125 | 12 450 |
| MODS culture and DST | 78 616 | 78 616 | 78 616 | 78 616 |
| NRA culture and DST | 52 083 | 52 083 | 52 083 | 52 083 |
| MTBDRplus detection and DST | 25 100 | 25 100 | 25 100 | 25 100 |
| Xpert MTB/RIF detection and rifampin DST | 6250 | 6250 | 6250 | 6250 |
Crude unit costs (capital outlay, maintenance, QA and human resources costs not considered here) for culture and RH DST that were used: LJ culture and proportion method DST at $2.29 per slope [12] = $2.29 × 5 (primary isolation followed by indirect DST with 2 control slopes plus separate R and H containing slopes) = $11.45 per patient; MGIT culture and DST at $5.02 per tube [12] = × 4 (primary isolation followed by indirect DST with 1 control tube plus separate R and H containing tubes) = $20.08 per patient; MODS culture and DST at $3.18 per patient [46]; NRA culture and DST at $4.80 per patient [47]; MTBDRplus detection and DST at $9.96 per patient [48]; Xpert MTB/RIF detection and DST at $40.00 per test (R. Rustomjee, personal communication).
Of whom 20% have culture-positive TB (half of which is smear-positive).
DST costs for LJ and MGIT are only included for positive cultures.
CONCLUDING THOUGHTS FOR PROGRAM MANAGERS
There is a clear imperative for improving diagnosis of drug-resistant TB in all low- and middle-income countries. An array of rapid diagnostic tests is available, with differing cost, logistical, and technical considerations. Patient characteristics are inadequate for predicting MDR-TB; thus, universal culture and DST for all persons with suspected TB must be made available. Programs should not be constrained by concerns about limited treatment capacity but, rather, should develop comprehensive national plans for MDR- and XDR-TB management that include Green Light Committee support and/or novel approaches to treatment delivery. There is significant and increasing evidence that MDR-TB treatment can be effectively delivered and that laboratory systems can be strengthened in even the poorest countries. There are lessons to be learned from and, perhaps, even infrastructure to be shared with HIV diagnosis and treatment services [49]. With increased funding available through the Global Fund, UNITAID, and President’s Emergency Plan for AIDS Relief for countries to improve diagnosis and surveillance for MDR-TB, together with technical assistance for laboratory scale-up through the Global Laboratory Initiative, national TB programs are poised to make the leap from the microscopy of the 1880s to the rapid diagnostics of today. They can no longer afford not to.
Notes
Financial support.
D. A. J. M. is supported by the Wellcome Trust and LSHTM. N. S. S. is supported by the Doris Duke Charitable Foundation Clinical Scientist Development Award and the President’s Emergency Plan for AIDS Relief.
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Chavez Pachas AM, Blank R, Smith Fawzi MC, Bayona J, Becerra MC, Mitnick CD. Identifying early treatment failure on category I therapy for pulmonary tuberculosis in Lima Ciudad, Peru. Int J Tuberc Lung Dis. 2004;8:52–8. [PubMed] [Google Scholar]
- 2.World Health Organization. Guidelines for the programmatic management of drug-resistant tuberculosis. Report No.: WHO/HTM/TB/2006.361. Geneva, Switzerland: World Health Organization, 2006. [Google Scholar]
- 3.Keshavjee S, Farmer PE. Picking up the pace—scale-up of MDR tuberculosis treatment programs. N Engl J Med. 2010;363:1781–4. doi: 10.1056/NEJMp1010023. [DOI] [PubMed] [Google Scholar]
- 4.Nathanson E, Lambregts-van WC, Rich ML, et al. Multidrug-resistant tuberculosis management in resource-limited settings. Emerg Infect Dis. 2006;12:1389–97. doi: 10.3201/eid1209.051618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wells CD, Cegielski JP, Nelson LJ, et al. HIV infection and multidrug-resistant tuberculosis: the perfect storm. J Infect Dis. 2007;196(Suppl 1):S86–107. doi: 10.1086/518665. [DOI] [PubMed] [Google Scholar]
- 6.Gandhi NR, Moll A, Sturm AW, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575–80. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- 7.Sotgiu G, Ferrara G, Matteelli A, et al. Epidemiology and clinical management of XDR-TB: a systematic review by TBNET. Eur Respir J. 2009;33:871–81. doi: 10.1183/09031936.00168008. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. Report No.: WHO/HTM/TB/2010.3. Geneva, Switzerland: World Health Organization, 2010. [Google Scholar]
- 9.Gandhi NR, Nunn P, Dheda K, et al. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet. 2010;375:1830–43. doi: 10.1016/S0140-6736(10)60410-2. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Guidelines for surveillance of drug resistance in tuberculosis. 4th ed. Report No.: WHO/HTM/TB/2009.422. Geneva, Switzerland: World Health Organization, 2009. [Google Scholar]
- 11.World Health Organization. The use of liquid TB culture and drug susceptibility testing (DST) in low and medium income settings. Geneva, Switzerland: World Health Organization. 2007. http://www.who.int/tb/dots/laboratory/policy/en/index3.html. Accessed 16 October 2010. [Google Scholar]
- 12.Pai M, Ramsay A, O'Brien R. Evidence-based tuberculosis diagnosis. PLoS Med. 2008;5:e156. doi: 10.1371/journal.pmed.0050156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Minion J, Leung E, Menzies D, Pai M. Microscopic-observation drug susceptibility and thin layer agar assays for the detection of drug resistant tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:688–98. doi: 10.1016/S1473-3099(10)70165-1. [DOI] [PubMed] [Google Scholar]
- 14.Ling DI, Zwerling AA, Pai M. GenoType MTBDR assays for the diagnosis of multidrug-resistant tuberculosis: a meta-analysis. Eur Respir J. 2008;32:1165–74. doi: 10.1183/09031936.00061808. [DOI] [PubMed] [Google Scholar]
- 15.Palomino JC. Molecular detection, identification and drug resistance detection in Mycobacterium tuberculosis. FEMS Immunol Med Microbiol. 2009;56:103–11. doi: 10.1111/j.1574-695X.2009.00555.x. [DOI] [PubMed] [Google Scholar]
- 16.Wallis RS, Pai M, Menzies D, et al. Biomarkers and diagnostics for tuberculosis: progress, needs, and translation into practice. Lancet. 2010;375:1920–37. doi: 10.1016/S0140-6736(10)60359-5. [DOI] [PubMed] [Google Scholar]
- 17.Minion J, Pai M. Bacteriophage assays for rifampicin resistance detection in Mycobacterium tuberculosis: updated meta-analysis. Int J Tuberc Lung Dis. 2010;14:941–51. [PubMed] [Google Scholar]
- 18.Kalantri S, Pai M, Pascopella L, Riley L, Reingold A. Bacteriophage- based tests for the detection of Mycobacterium tuberculosis in clinical specimens: a systematic review and meta- analysis. BMC Infect Dis. 2005;5:59. doi: 10.1186/1471-2334-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin A, Portaels F, Palomino JC. Colorimetric redox-indicator methods for the rapid detection of multidrug resistance in Mycobacterium tuberculosis: a systematic review and meta-analysis. J Antimicrob Chemother. 2007;59:175–83. doi: 10.1093/jac/dkl477. [DOI] [PubMed] [Google Scholar]
- 20.Martin A, Panaiotov S, Portaels F, Hoffner S, Palomino JC, Angeby K. The nitrate reductase assay for the rapid detection of isoniazid and rifampicin resistance in Mycobacterium tuberculosis: a systematic review and meta-analysis. J Antimicrob Chemother. 2008;62:56–64. doi: 10.1093/jac/dkn139. [DOI] [PubMed] [Google Scholar]
- 21.Moore DA, Evans CA, Gilman RH, et al. Microscopic-observation drug-susceptibility assay for the diagnosis of TB. N Engl J Med. 2006;355:1539–50. doi: 10.1056/NEJMoa055524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arias M, Mello FC, Pavon A, et al. Clinical evaluation of the microscopic-observation drug-susceptibility assay for detection of tuberculosis. Clin Infect Dis. 2007;44:674–80. doi: 10.1086/511639. [DOI] [PubMed] [Google Scholar]
- 23.Shiferaw G, Woldeamanuel Y, Gebeyehu M, Girmachew F, Demessie D, Lemma E. Evaluation of microscopic observation drug susceptibility assay for detection of multidrug-resistant Mycobacterium tuberculosis. J Clin Microbiol. 2007;45:1093–7. doi: 10.1128/JCM.01949-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mello FC, Arias MS, Rosales S, et al. Clinical evaluation of the microscopic observation drug susceptibility assay for detection of Mycobacterium tuberculosis resistance to isoniazid or rifampin. J Clin Microbiol. 2007;45:3387–9. doi: 10.1128/JCM.00580-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ejigu GS, Woldeamanuel Y, Shah NS, Gebyehu M, Selassie A, Lemma E. Microscopic-observation drug susceptibility assay provides rapid and reliable identification of MDR-TB. Int J Tuberc Lung Dis. 2008;12:332–7. [PubMed] [Google Scholar]
- 26.Tovar M, Siedner MJ, Gilman RH, et al. Improved diagnosis of pleural tuberculosis using the microscopic-observation drug-susceptibility technique. Clin Infect Dis. 2008;46:909–12. doi: 10.1086/527447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ha DT, Lan NT, Wolbers M, et al. Microscopic observation drug susceptibility assay (MODS) for early diagnosis of tuberculosis in children. PLoS One. 2009;4:e8341. doi: 10.1371/journal.pone.0008341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bwanga F, Hoffner S, Haile M, Joloba ML. Direct susceptibility testing for multi drug resistant tuberculosis: a meta-analysis. BMC Infect Dis. 2009;9:67. doi: 10.1186/1471-2334-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morgan M, Kalantri S, Flores L, Pai M. A commercial line probe assay for the rapid detection of rifampicin resistance in Mycobacterium tuberculosis: a systematic review and meta-analysis. BMC Infect Dis. 2005;5:62. doi: 10.1186/1471-2334-5-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnard M, Albert H, Coetzee G, O'Brien R, Bosman ME. Rapid molecular screening for multidrug-resistant tuberculosis in a high-volume public health laboratory in South Africa. Am J Respir Crit Care Med. 2008;177:787–92. doi: 10.1164/rccm.200709-1436OC. [DOI] [PubMed] [Google Scholar]
- 31.Nikolayevsky V, Brown T, Balabanova Y, Ruddy M, Fedorin I, Drobniewski F. Detection of mutations associated with isoniazid and rifampin resistance in Mycobacterium tuberculosis isolates from Samara Region, Russian Federation. J Clin Microbiol. 2004;42:4498–502. doi: 10.1128/JCM.42.10.4498-4502.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miotto P, Piana F, Cirillo DM, Migliori GB. Genotype MTBDRplus: a further step toward rapid identification of drug-resistant Mycobacterium tuberculosis. J Clin Microbiol. 2008;46:393–4. doi: 10.1128/JCM.01066-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hillemann D, Weizenegger M, Kubica T, Richter E, Niemann S. Use of the genotype MTBDR assay for rapid detection of rifampin and isoniazid resistance in Mycobacterium tuberculosis complex isolates. J Clin Microbiol. 2005;43:3699–703. doi: 10.1128/JCM.43.8.3699-3703.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hillemann D, Rusch-Gerdes S, Richter E. Application of the Genotype MTBDR assay directly on sputum specimens. Int J Tuberc Lung Dis. 2006;10:1057–9. [PubMed] [Google Scholar]
- 35.Evans J, Stead MC, Nicol MP, Segal H. Rapid genotypic assays to identify drug-resistant Mycobacterium tuberculosis in South Africa. J Antimicrob Chemother. 2009;63:11–6. doi: 10.1093/jac/dkn433. [DOI] [PubMed] [Google Scholar]
- 36.Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–15. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization. Frequently Asked Questions on XPert® MTB/RIF assay. Geneva, Switzerland: World Health Organization; 2011. http://www.who.int/tb/laboratory/xpert_faqs.pdf. Accessed 10 May 2011. [Google Scholar]
- 38.Foundation for Innovative New Diagnostics. FIND negotiated proces for Xpert® MTB/RIF and country list. Geneva, Switzerland: FIND; 2011. http://www.finddiagnostics.org/programs/find-negotiated-prices/mtbdrplus.html. Accessed 10 May 2011. [Google Scholar]
- 39.Dowdy DW, Lourenco MC, Cavalcante SC, et al. Impact and cost-effectiveness of culture for diagnosis of tuberculosis in HIV-infected Brazilian adults. PLoS One. 2008;3:e4057. doi: 10.1371/journal.pone.0004057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chihota VN, Grant AD, Fielding K, et al. Liquid vs. solid culture for tuberculosis: performance and cost in a resource-constrained setting. Int J Tuberc Lung Dis. 2010;14:1024–31. [PubMed] [Google Scholar]
- 41.cuna-Villaorduna C, Vassall A, Henostroza G, et al. Cost-effectiveness analysis of introduction of rapid, alternative methods to identify multidrug-resistant tuberculosis in middle-income countries. Clin Infect Dis. 2008;47:487–95. doi: 10.1086/590010. [DOI] [PubMed] [Google Scholar]
- 42.Small PM. Strengthening laboratory services for today and tomorrow. Plenary Lecture given during the 38th Union World Lung Conference on Lung Health, Cape Town, South Africa, 8–12 November 2007. Int J Tuberc Lung Dis. 2008;12:1105–9. [PubMed] [Google Scholar]
- 43.Gandhi NR, Shah NS, Andrews JR, et al. HIV coinfection in multidrug- and extensively drug-resistant tuberculosis results in high early mortality. Am J Respir Crit Care Med. 2010;181:80–6. doi: 10.1164/rccm.200907-0989OC. [DOI] [PubMed] [Google Scholar]
- 44.Nardell E, Dharmadhikari A. Turning off the spigot: reducing drug-resistant tuberculosis transmission in resource-limited settings. Int J Tuberc Lung Dis. 2010;14:1233–43. [PMC free article] [PubMed] [Google Scholar]
- 45.Keshavjee S, Gelmanova IY, Farmer PE, et al. Treatment of extensively drug-resistant tuberculosis in Tomsk, Russia: a retrospective cohort study. Lancet. 2008;372:1403–9. doi: 10.1016/S0140-6736(08)61204-0. [DOI] [PubMed] [Google Scholar]
- 46.Shah NS, Richardson JR, Moodley P, et al. Increasing drug resistance in extensively drug-resistant tuberculosis, South Africa. Emerg Infect Dis. 2009;17:510–3. doi: 10.3201/eid1703.101363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buthelezi SSS. Program and abstracts of the 2nd Meeting of the Global XDR TB Task Force. Geneva, Switzerland: World Health Organization; 2008. Situational analysis of TB drug resistance in KwaZulu-Natal Province: Republic of South Africa. [Google Scholar]
- 48.Brust JCM, Moll A, Scott M, et al. Program and abstracts of the XVII International AIDS Conference (Mexico City, Mexico) Geneva, Switzerland: International AIDS Society; 2009. Community-based treatment of multidrug-resistant tuberculosis (MDR TB) and HIV in rural South Africa. [Google Scholar]
- 49.IOM (Institute of Medicine) Addressing the threat of drug-resistant tuberculosis: a realistic assessment of the challenge: workshop summary. Washington, DC: National Academies Press; 2009. [PubMed] [Google Scholar]
- 50.Griffin AM, Caviedes L, Gilman R, et al. Field and laboratory preparedness: challenges to rolling out new multidrug-resistant tuberculosis diagnostics. Rev Panam Salud Publica. 2009;26:120–7. doi: 10.1590/s1020-49892009000800004. [DOI] [PubMed] [Google Scholar]