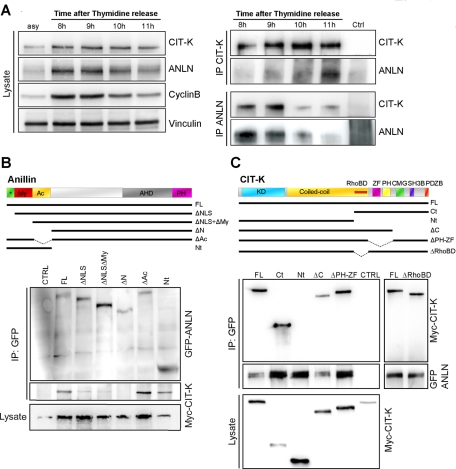
FIGURE 4:
CIT-K forms a physical complex with anillin. (A) HeLa cells were synchronized at the G1/S boundary by a thymidine block, released into fresh media, and harvested every hour. Left, Total cell extracts from asynchronous cells (asy) or from the indicated time points were analyzed by Western blotting to monitor the amount of CIT-K and anillin around the time of mitosis exit (corresponding to 11 h, as shown by cyclincyclin B decrease). Vinculin was used as internal loading control. Right, Total extracts from the indicated time points were immunoprecipitated with anti-CIT-K or anti-anillin antibodies. Western blotting with the indicated antibodies revealed reciprocal coimmunoprecipitation of the endogenous proteins. (B) The indicated GFP-anillin fusion constructs were cotransfected with Myc-tagged CITK-expression plasmid in HEK293T for 48 h. Immunoprecipitations were then performed from total cell lysates using anti-GFP antibody, and Western blotting was used to reveal the immunoprecipitated proteins for the GFP and Myc epitopes. Conserved regions and previously described domains are indicated: *, 2 × NLS sequences; My, myosin-binding domain (aa 146–258); Ac, actin-binding and -bundling region (aa 258–371); AHD, anillin homology domain (aa 608–943); PH domain (aa 943–1087). (C) The indicated Myc-CIT-K fusion constructs were cotransfected with GFP-anillin, as in (B). Immunoprecipitations and Western blotting were performed as in (B). Conserved regions and previously described domains are indicated: KD, Ser/Thr kinase domain; Coiled-coil, coiled-coil region; RhoBD, Rho-binding domain; ZF domain; PH domain; CMG; SH3B domain; PDZB domain. In every case, the results shown are representative examples of at least three different experiments.