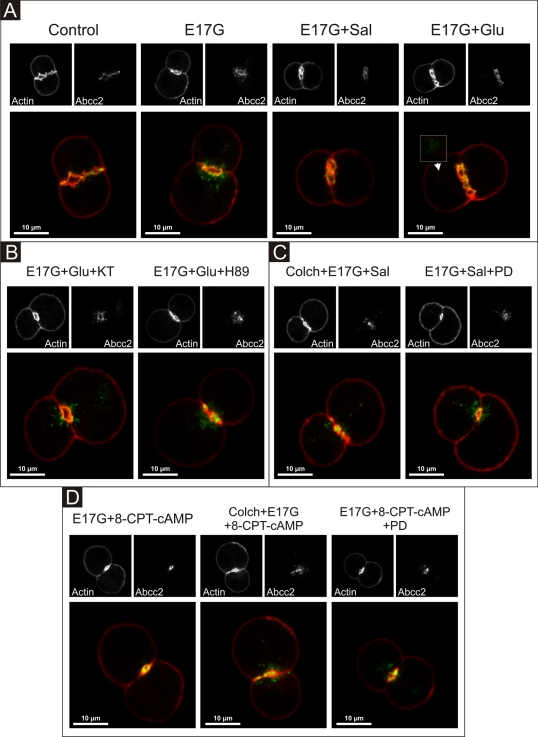
FIGURE 11:
Representative confocal images showing cellular distribution of Abcc2, actin, and merged images (with actin in red and Abcc2 in green) in IRHCs under different treatments. (A) Prevention by Glu (0.1 μM) and Sal (1 μM) of E17G (200 μM)-induced retrieval of Abcc2. Note that under control conditions Abcc2-associated fluorescence is mainly localized at the canalicular membrane in the area delimited by the pericanalicular actin ring. E17G induced a clear internalization of Abcc2-containing vesicles beyond the limits of the pericanalicular actin ring, a phenomenon significantly prevented by Glu and Sal. The arrowhead in the E17G + Glu group shows some transporter-containing vesicles that remain internalized in a deep intracellular compartment. (B) Preincubation with the PKA inhibitor H-89 (200 nM) or KT5720 (KT, 50 nM) significantly inhibited the preventive effect of Glu on E17G-induced internalization of Abcc2. (C) Pretreatment of IRHCs with the microtubule-disrupting agent colchicine (Colch, 1 μM) or the MEK1/2 inhibitor PD98059 (PD, 1 μM) abolished the preventive effect of Sal on E17G-induced internalization of Abcc2. (D) Pretreatment of IRHCs with 8-CPT-2´-O-Me-cAMP (8-CPT-cAMP, 50 μM), an Epac agonist, also prevented delocalization of Abcc2 induced by E17G, a phenomenon also abolished by preincubation with Colch and PD. None of the treatments affected the normal distribution of actin, which showed a predominant pericanalicular distribution.