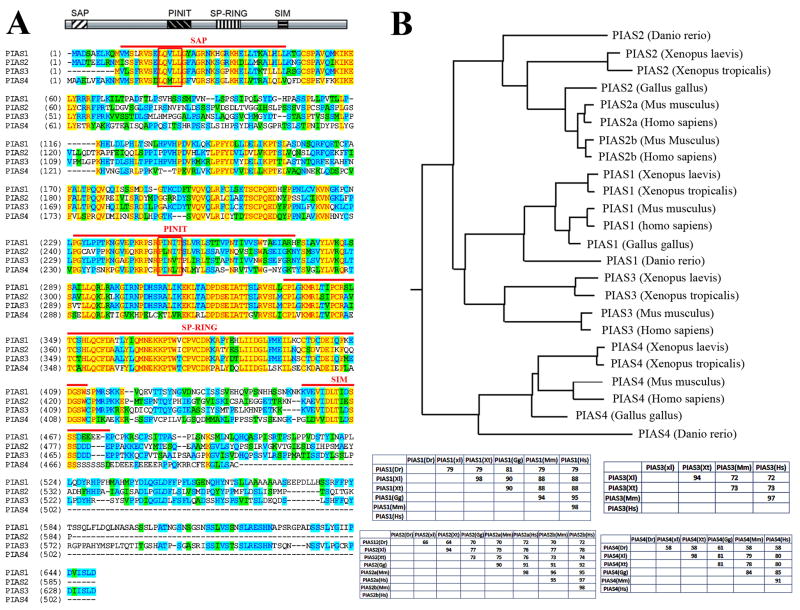
Figure 1. PIAS proteins are conserved across the vertebrate species.
A) Alignment of the four Xenopus laevis PIAS proteins reveals that they share a high degree of homology within the known functional domains, including the SAP, PINIT, SP-RING and SIM domains (marked by the red lines above the sequences), but are more divergent in the carboxyl termini. The LXXLL motif in the SAP domain and the PINIT motif in the PINIT domain are marked by the closed boxes. B) Alignment of vertebrate PIAS proteins show that PIAS are highly conserved in vertebrate. The phylogenic tree constructed using the neighbor-joining algorithm is shown here, and the identity tables for each vertebrate PIAS protein are shown at the bottom.