Figure 1.
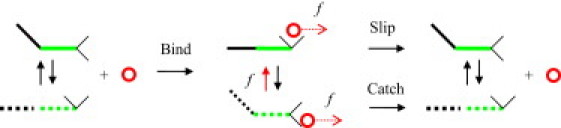
Binding and dissociation of a receptor/ligand catch-bond complex. The diagram is based most directly on the FimH/mannose system (27,44); however, it also represents other catch-bonds. The two-domain fragment of the receptor protein, composed of the pilin (black) and lectin (green) domains, exists in either bent or extended state. (Solid lines) More stable conformation; (dashes) less stable conformation. The bent state is more favorable in the free receptor. Binding to mannose (circle) shifts the conformational equilibrium toward the extended state. The interaction between lectin's binding site (angle made of two thin black lines) and mannose is stronger for the extended state (angle holding the circle) than the bent state (angle releasing the circle). The applied force (red arrows) lowers the bond dissociation barrier and favors the extended conformation. The control of the receptor conformation by force, combined with the correlation between the receptor conformation and the receptor/ligand binding strength, forms the basis for the allosteric model (19); see also Fig. 2a. The bond dissociations via the bent and extended channels represent the catch- and slip-pathways in the two-pathway model (12); see also Fig. 2b.
