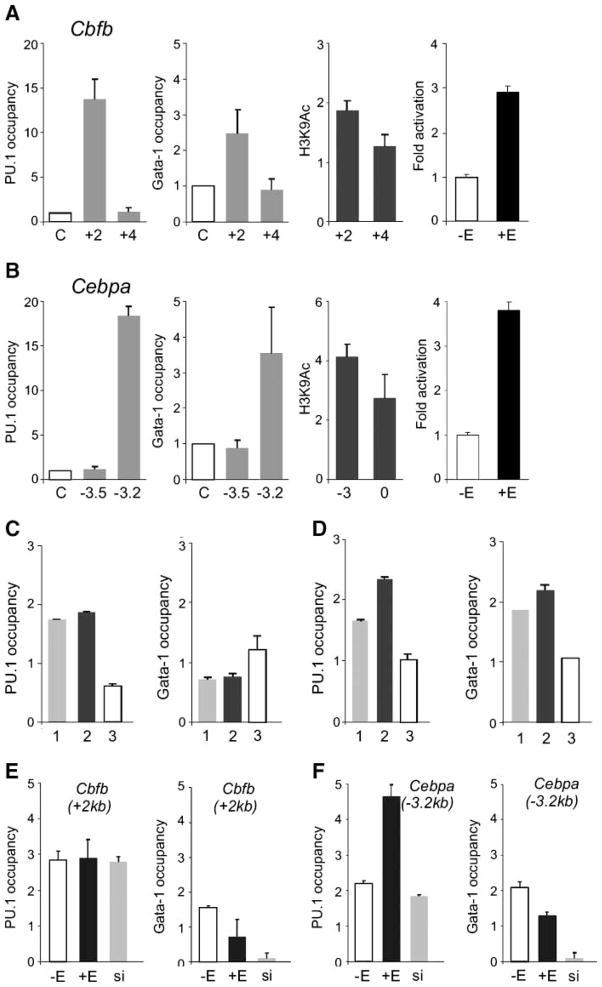
FIGURE 4.
PU.1 and GATA-1 colo-calization near Cbfb and Cebpa genes and chromatin H3K9 hyperacetylation induced by ectopic PUER activation. ChIP was carried out on cross-linked chromatin as described in Materials and Methods using the following antibodies: anti-PU.1, anti–GATA-1 (both gray columns), anti–acetylated histone H3K9 (black columns), and control anti-rabbit IgG antibody (white columns, letter C on the X-axis). A and B. Occupancy of PU.1 and GATA-1 proteins at indicated positions (relative to transcription start site, in kilobases) near Cbfb and Cebpa was determined in unstimulated MELPUER cells. Levels of H3K9 hyperacetylation in these cells in the presence of 10−7 mol/L of 17β-estradiol for 24 h (third graphs in A and B) was determined relative to acetylation determined in stimulated MELGER cells under the same conditions. Primary binding sites of PU.1 were functional in reporter assays: 1.4 × 105 MELPUER cells were lipofected with Cbfb(+1531) (A, right) or Cebpa(−2978) (B, right) reporter plasmids (1.7 μg each). Cells remained unstimulated (−E) or treated with 17β-estradiol at 24 h (+E). Luciferase activity was determined 72 h after trans-fection (for details, see Materials and Methods). C and D. DNA regions (50–60 bp) near Cebpa(−2978) (no. 1 on X-axis) and Cbfb(+1531) (no. 2) were cloned together with a Cebpa(−2978) mutant (no. 3) into the reporter plasmid pGL3 and transfected into MELPUER (C) and MELGER (D) cells stimulated with 17β-estradiol for 72 h. Transfected qChIP technique using antibodies to PU.1, GATA-1, and antirabbit IgG antibody was done as described in Materials and Methods. PU.1 significantly stimulated luciferase activity of PU.1 binding site nos. 1 and 2, but not in site no. 3 (data not shown). The occupancy of PU.1 near Cebpa (−3.2 kb; E) and Cbfb (+2 kb; F) genes was tested by ChIP in either stimulated (48 h; +E) or unstimulated (−E) MELPUER cells and in MELPUER cells with knockdown of GATA-1 (si). All ChIP bars in C to F indicate the fold change (Y-axis) of DNA fragment in specific immunoprecipitates above the immunoprecipitates using control antirabbit IgG antibody. Bars, SE of at least two independent experiments.