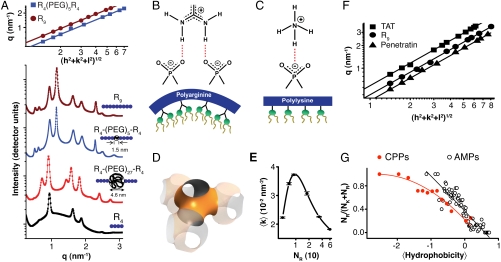
Fig. 2.
Membrane activity of CPPs controlled by lipid crowding effects and amino acid content. (A) SAXS data for R9, incubated with SUVs (DOPS∶DOPE = 20∶80) at peptide-to-lipid molar ratio (P/L) = 1/40 show diffraction peak positions well described by 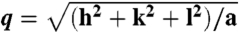 , characteristic of a bicontinuous cubic Pn3m phase (h, k, l are Miller indices; a is the lattice parameter). Tetraarginine blocks connected with a short PEG spacer (R4-(PEG)5-R4) induces a Pn3m phase (P/L = 1/40) with a larger lattice constant. SAXS data for R4-(PEG)27-R4, with a longer PEG spacer, show that the Pn3m phase is suppressed, replaced by HII and Lα phases with no saddle-splay curvature (P/L = 1/40). (B) Multidentate coordination of arginine’s guanidinium side chain induces positive curvature strain along the peptide. (C) Monodentate coordination of lysine’s amino side chain does not induce positive curvature. (D) The cubic Pn3m phase (the zero-mean-curvature surface at the midplane between the two membrane leaflets) (E) The average induced Gaussian curvature
, characteristic of a bicontinuous cubic Pn3m phase (h, k, l are Miller indices; a is the lattice parameter). Tetraarginine blocks connected with a short PEG spacer (R4-(PEG)5-R4) induces a Pn3m phase (P/L = 1/40) with a larger lattice constant. SAXS data for R4-(PEG)27-R4, with a longer PEG spacer, show that the Pn3m phase is suppressed, replaced by HII and Lα phases with no saddle-splay curvature (P/L = 1/40). (B) Multidentate coordination of arginine’s guanidinium side chain induces positive curvature strain along the peptide. (C) Monodentate coordination of lysine’s amino side chain does not induce positive curvature. (D) The cubic Pn3m phase (the zero-mean-curvature surface at the midplane between the two membrane leaflets) (E) The average induced Gaussian curvature 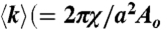 ; χ = -2 and Ao = 1.919 for Pn3m) is maximized near NR = 9, where observed membrane transduction activity is empirically highest. (F) ANTP, TAT peptide, R9 all induce the cubic Pn3m phase in membranes (DOPS∶DOPE = 20∶80 at P/L = 1/40). (G) The ratio of the number of arginines to the number of arginines + number of lysines (NR/(NR + NK)) plotted against hydrophobicity (Eisenberg hydrophobicity scale) using the amino acid sequences of 39 cell-penetrating peptides and 1080 cationic antimicrobial peptides shows that a reduction in arginine content can be compensated for by an increase in both lysine and hydrophobic content.
; χ = -2 and Ao = 1.919 for Pn3m) is maximized near NR = 9, where observed membrane transduction activity is empirically highest. (F) ANTP, TAT peptide, R9 all induce the cubic Pn3m phase in membranes (DOPS∶DOPE = 20∶80 at P/L = 1/40). (G) The ratio of the number of arginines to the number of arginines + number of lysines (NR/(NR + NK)) plotted against hydrophobicity (Eisenberg hydrophobicity scale) using the amino acid sequences of 39 cell-penetrating peptides and 1080 cationic antimicrobial peptides shows that a reduction in arginine content can be compensated for by an increase in both lysine and hydrophobic content.