Abstract
The dormant state known as diapause is widely exploited by insects to circumvent winter and other adverse seasons. For an insect to survive, feed, and reproduce at the appropriate time of year requires fine coordination of the timing of entry into and exit from diapause. One of the hormones that regulates diapause in moths is the 24-aa neuropeptide, diapause hormone (DH). Among members of the Helicoverpa/Heliothis complex of agricultural pests, DH prompts the termination of pupal diapause. Based on the structure of DH, we designed several agonists that are much more active than DH in breaking diapause. One such agonist that we describe also prevents the entry into pupal diapause when administered to larvae that are environmentally programmed for diapause. In addition, we used the unique antagonist development strategy of incorporating a dihydroimidazole (“Jones”) trans-Proline mimetic motif into one of our DH agonists, thereby converting the agonist into a DH antagonist that blocks the termination of diapause. These results suggest potential for using such agents or next-generation derivatives for derailing the success of overwintering in pest species.
Keywords: diapause manipulation, peptidomimetics
Coordinating active phases of the life cycle with seasons that provide food resources and suitable environmental conditions is crucial for sustaining viable insect populations. Major portions of the year, most notably winters in temperate zones, are unsuitable for continuous development, and most insects have evolved periods of dormancy (diapause), characterized by suppressed metabolism and bolstered stress responses that enhance survival during unfavorable seasons. Short day lengths of late summer commonly trigger the onset of diapause (1, 2), and these environmental signals prompt endocrine responses that directly initiate and eventually terminate the diapause state (3).
Desynchronizing an insect pest with its appropriate seasonal diapause could be used as a tool for disrupting pest populations (4). Altering the timing of diapause has the potential to evoke ecological suicide if the insect is forced to be active during a time of year when climatic conditions are adverse or food resources are absent. Blocking entry into diapause in the autumn, breaking out of an overwintering diapause prematurely, or failing to terminate diapause at the appropriate time in the spring all have potential for desynchronizing the temporal distribution of insects. We report here the development of unique agents capable of disrupting the overwintering pupal diapause of the corn earworm, Helicoverpa zea, a member of the Heliothis/Helicoverpa complex, a worldwide group of noteworthy crop pests (5).
Our target is diapause hormone (DH). This 24-aa neuropeptide, a pyrokinin in the DH-pheromone biosynthesis activating neuropeptide (PBAN) family, is best known for its action in initiating embryonic diapause in the commercial silkworm, Bombyx mori (6); however, recently, DH was also shown to exert an opposite effect in pupae of the Heliothis/Helicoverpa complex. Rather than inducing diapause as it does in B. mori, DH actually breaks diapause in Heliothis/Helicoverpa (7–10). Based on the active core of the native hormone, we report the development of several hyperpotent agonists of DH capable of terminating diapause as well as preventing entry into diapause. In addition, we report an antagonist of DH, an agent with a unique structure that blocks the action of DH in terminating diapause. The rationally developed peptide agonists and antagonist that we report here offer previously undescribed tools that could be directed against this group of agriculturally important pests.
Results and Discussion
The corn earworm, H. zea, like other members of this pest complex, enters an overwintering diapause in the pupal stage in response to short day lengths and low temperatures of autumn (11). We previously established that diapause in pupae of these moths can be terminated with an injection of DH, a neuropeptide produced in the subesophageal ganglion. DH works together with another neuropeptide, prothoracicotropic hormone, to promote synthesis of the ecdysteroids needed to break diapause and initiate adult development. Although both peptides normally contribute to the termination of diapause, either one by itself is fully capable of breaking diapause at suitable temperatures (12). Based on the structure of DH, we previously reported several agonists capable of terminating diapause (10). The results we report here describe several unique highly potent agonists capable of breaking diapause; we demonstrate that certain of these agonists can be applied to larvae, and thus prevent the entry into pupal diapause. In addition, we report the development of a potent DH antagonist capable of blocking the termination of diapause. We thus present chemical tools capable of breaking diapause, preventing the entry into diapause, and blocking the exit from diapause. Sequences of the peptide agonists and antagonists discussed here are presented in Table S1.
Breaking Pupal Diapause with DH Agonists.
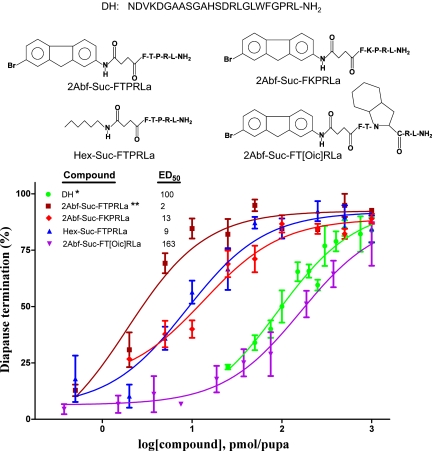
Previously, we reported that the active core of DH in H. zea consists of seven amino acids located at the C terminus of the native DH. The ED50 for DH in terminating diapause is ∼100 pmol per pupa, as shown in Fig. 1. The most potent agonist we developed, PK–2-amino-7-bromo-fluorene (2Abf)–succinoyl (Suc)-FTPRLa, has an ED50 of 2 pmol per pupa, indicating that it is ∼50-fold more potent in breaking diapause than the native DH. Based on this lead molecule, structure-modified compounds, including 2Abf-Suc-FKPRLa (DH-2Abf-K), hexylamine (Hex)-Suc-FTPRLa, and 2Abf-Suc-FT[octahydroindole-2-carboxyl (Oic)]RLa, were designed and synthesized. The dose–response curves for DH-2Abf-K and the two related analogs (Fig. 1) were compared with curves for DH and the agonist PK-2Abf, which was previously reported (9, 10). The ED50 of DH-2Abf-K was 13 pmol, a value indicating that this agonist is far more potent than DH but not quite as active as PK-2Abf. The difference in efficacy of DH-2Abf-K and PK-2Abf indicates that the amino acid in the variable position (X in FXPRLamide) plays an important role for diapause termination.
Fig. 1.
Termination of pupal diapause in H. zea in response to DH and four agonists, whose structures are shown. ED50s are expressed in picomoles per pupa, and each compound was evaluated in a range of doses from 0 to 1,000 pmol per pupa. Error bars indicate SDs from three replicates. A total of 14–20 pupae were tested in each replicate. *Data from Zhang et al. (9). **Data from Zhang et al. (10).
When Hex replaced the 2Abf portion of PK-2Abf, the ED50 of the newly developed compound increased nearly fourfold, and when the bulky Proline (Pro) analog Oic was substituted for Pro, the ED50 of the newly developed compound increased dramatically to 163 pmol, making the compound much less potent than DH. This is consistent with our previous study showing that both components of the “biophore” (the hydrophobic 2Abf and the trans-oriented Pro structure) and the associated β-turn are essential for diapause termination (10). The results demonstrate again that a slight modification at these positions has a significant impact on the activity of these compounds.
Thus, the most potent agonists containing a 2Abf motif and capable of terminating diapause are PK-2Abf and DH-2Abf-K. With ED50s that are significantly lower than that of native DH, both pseudopeptides have the potential to serve as agents for prematurely breaking pupal diapause in H. zea.
Preventing Pupal Diapause with DH Agonists Administered to Larvae.
This set of experiments was designed to identify compounds that could be applied to larvae and subsequently prevent the entry into pupal diapause. Although the potent DH agonists discussed above are quite effective in terminating diapause, the diapause stage itself may present a challenge for application because diapausing pupae are already underground, and hence remain less accessible to any sort of chemical treatment. A much more accessible stage for applying a chemical agent would be the larval stage, which is still above ground. For these experiments, larvae were reared at 21 °C with a photoperiod of 8 h light (L)/16 h dark (D), conditions that yielded a pupal diapause incidence of ∼60%. Larvae late in the final larval instar were injected with candidate compounds, and we then evaluated the capacity for these compounds to prevent entry into pupal diapause.
We first tested PK-2Abf, the most potent DH agonist discussed above, and related compounds. The hyperpotency of PK-2Abf and the increased half-life generated by blocking the N terminus with the hydrophobic 2Abf–Suc complex suggested that it might be capable of being administered to larvae and retain its potency long enough to prevent diapause entry. Using PK-2Abf as the lead molecule, nine more compounds were designed (Fig. 2A). Four analogs with amino acid residue replacements in the variable X position (PK-2Abf: X = T) found in naturally occurring members of the pyrokinin/PBAN superfamily (X = A, G, K, S, T, V) were tested. Other analogs tested featured modifications of the more critical nonvariant residues in the core region.
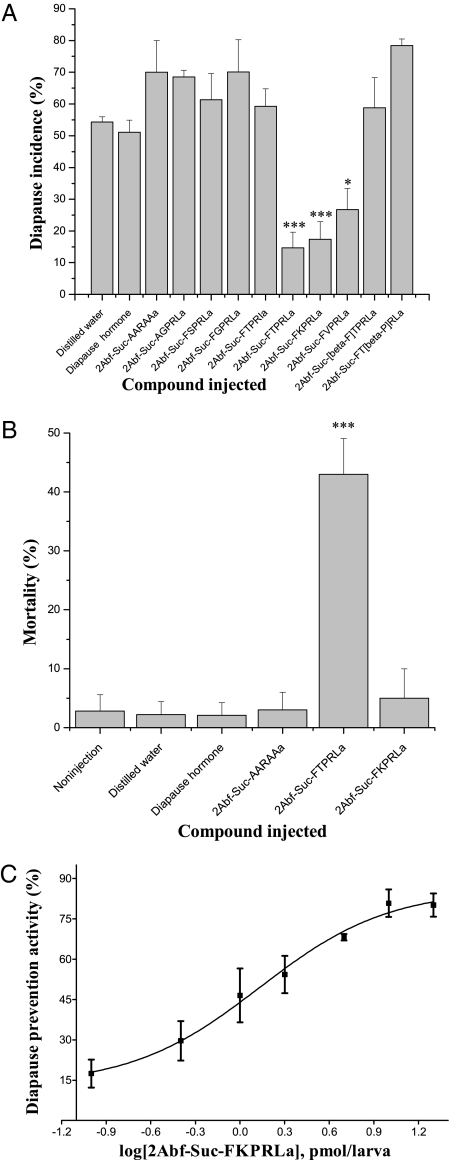
Fig. 2.
Screening of potential diapause prevention agents indicates that three peptide analogs, 2Abf-Suc-FTPRLa (PK-2Abf), 2Abf-Suc-FKPRLa (DH-2Abf-K), and 2Abf-Suc-FVPRLa, are capable of preventing diapause (A) but that PK-2Abf also causes significant mortality (B). DH was tested at a dose of 1 nmol, whereas the other compounds were tested at a dose of 100 pmol. (C) Dose–response curve for DH-2Abf-K (structure in Fig. 1), the most potent agonist for preventing diapause. Error bars indicate SDs from three replicates; 12–20 larvae were tested in each replicate. The ED50 was ∼7 pmol per larva. Differences between the tested compound and distilled water are shown by asterisks (Student t test: *P < 0.05; ***P < 0.001).
Controls injected with distilled water displayed a diapause incidence of 54%. Three compounds, including PK-2Abf, DH-2Abf-K, and 2Abf-Suc-FVPRLa, showed significant diapause prevention activity, with a diapause incidence of 15%, 17%, and 27%, respectively. The difference between distilled water controls and either of the first two compounds was very significant (t test, P < 0.001). Seven other compounds, including 2Abf-Suc-FSPRLa, 2Abf-Suc-FGPRLa, 2Abf-Suc-AGPRLa, 2Abf-Suc-FTPRIa, 2Abf-Suc-AARAAa, 2Abf-Suc-FT[β3P]RLa, and 2Abf-Suc-[β3P]TPRLa, were not effective. DH (1 nmol per larva) was also injected into larvae, and although it is potent in breaking pupal diapause, it had no effect in preventing the entry into pupal diapause when injected into larvae. It is likely that DH is rapidly degraded by aminopeptidases and/or endopeptidases in the larval hemolymph, thus losing its activity before it can exert an effect on diapause, whereas PK-2Abf and DH-2Abf-K may retain activity sufficiently long to exert an effect on pupal diapause. In summary, PK-2Abf and DH-2Abf-K, the most potent of the compounds tested, are peptide hormone analogs shown to be capable of preventing diapause.
The inactivity of 2Abf-Suc-AARAAa, 2Abf-Suc-AGPRLa, and 2Abf-Suc-FTPRIa was likely attributable to modification of critical portions of the active core of the peptide. The results demonstrate that activity is clearly dependent on the presence of the intact pyrokinin core sequence FXPRLa. Surprisingly, neither 2Abf-Suc-FGPRLa nor 2Abf-Suc-FSPRLa showed diapause prevention activity, suggesting that even the variable amino acid residue in the FXPRLamide peptide family can exert a profound influence on the activity of this hydrophobic series of DH analogs in the diapause prevention assay.
Although PK-2Abf was highly effective in preventing diapause, it also caused 43% mortality at a dose of 100 pmol per larva (Fig. 2B), a mortality incidence significantly higher than observed in uninjected controls (2.8%) or in larvae injected with distilled water (2.2%). By contrast, injection of DH-2Abf-K caused only 5% mortality at the same dose, a value that did not significantly differ from those of larva injected with distilled water or uninjected controls. Very low mortality was also observed for DH (2.1%) at a dose of 1 nmol per larva or for the control compound 2Abf-Suc-AARAAa at a dose of 100 pmol per larva, as shown in Fig. 2B. High mortality for PK-2Abf was observed even at a dose as low as 2 pmol per larva (25%). Thus, PK-2Abf and DH-2Abf-K both prevented diapause, and although they differ by only a single amino acid, only PK-2Abf caused high mortality. The reason for mortality caused by PK-2Abf is not yet clear, but high mortality was also observed when PK-2Abf was utilized in a pheromonotropic assay using adult females of a closely related species, Heliothis virescens (13).
The efficacy of DH-2Abf-K (structure shown in Fig. 1) in preventing diapause, without causing mortality, prompted further characterization of this response. The ED50 for DH-2Abf-K on diapause prevention was ∼7 pmol per larva (Fig. 2C), an ED50 similar to that observed for PK-2Abf (4 pmol per larva). Diapause prevention activity of DH-2Abf-K increased progressively within the range of 0.5–25 pmol and reached the highest levels at 50–100 pmol per pupa.
The accessibility of the above-ground prediapause larvae makes this stage a particularly good target for control efforts. Although the compounds described here were administrated only by injection, we hope to make additional modifications that will facilitate oral and/or topical application.
Blocking Diapause Termination with a DH Antagonist.
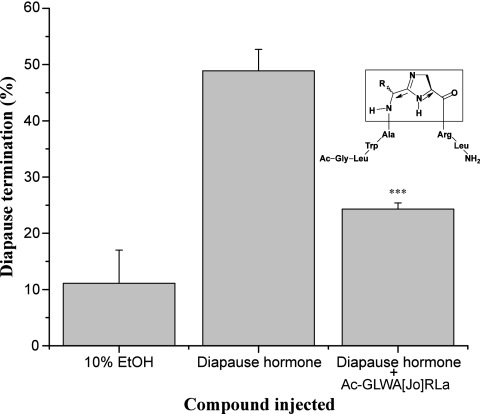
The final goal was to design DH antagonists capable of blocking the termination of diapause. Such agents could delay or completely block the natural processes of diapause termination, thus preventing the insect from exploiting favorable seasons for development and reproduction. Our approach was to target the DH activity core and convert the agonist into an antagonist molecule by modification of the structural components important for activity. Thirteen rationally designed antagonist candidates were evaluated (Table S1), but only a single candidate, acetyl (Ac)-GLWA[Jo]RLa (DH-Jo), showed a significant inhibitory effect when coinjected with DH (Fig. 3). As reported earlier, the ED50 for DH on diapause termination is ∼100 pmol per pupa (49% diapause termination incidence, as shown in Fig. 3). When 2 nmol of DH-Jo was coinjected with 100 pmol of DH, the diapause termination incidence dropped to 24% (t test, P < 0.001).
Fig. 3.
Structure and antagonistic activity of DH-Jo on diapause termination. Diapausing pupae were coinjected with 100 pmol of DH and 2 nmol of antagonist candidates dissolved in 10% (vol/vol) ethanol (EtOH). EtOH- and DH-injected pupae were used as negative and positive controls, respectively. Error bars indicate SDs from three replicates; 14–16 larvae were tested in each replicate. A very significant difference between the tested compounds and DH is shown by asterisks (Student t test: ***P < 0.001). Arrows on the structure of DH-Jo denote that the Jones motif mimics a trans-oriented peptide bond, including a transPro configuration.
Compound DH-Jo contains a dihydroimidazole motif (“Jones” motif) (14), which has been shown to serve as a surrogate for the trans-Pro conformation (Fig. 3) located in the activity core of pyrokinin/PBAN family peptides (15, 16). This compound was modified from the C-terminal heptapeptide active core (LWFGPRLa) of DH, which was shown to be active in the diapause termination assay (9). The amino acids GP were replaced by the Jones motif. A further modification was introduced by replacing F with A, the rationale being that any residual agonist activity would be eliminated by removal of the aromatic side chain of the F residue that is critical for the agonist response. The Jones motif is not as effective a mimic of a trans-oriented peptide bond as is the (E)-alkene moiety in the Etzkorn motif, as illustrated in numerous bioassays performed with peptides in the pyrokinin/PBAN family (16). However, in this study, the analog DH-Jo has proven to be an effective antagonist of DH. Possibly, this compound (DH-Jo) with a Jones motif can mimic the transPro configuration to an extent sufficient to bind the DH receptor but is unable to elicit the downstream response. This compound is an antagonist developed for DH and represents a unique class of peptide hormone antagonists. It is an example of an analog that incorporates a Jones trans-peptide bond/transPro mimic to generate an antagonistic response. This strategy may prove useful for the design of antagonists for other peptide hormones, especially those containing a transPro in the active core.
The primary method utilized previously to develop antagonists to the pyrokinin/PBAN family was to use a D-Phe substitution (17, 18), such as found in the PBAN antagonist PPK-AA (18). Another method for antagonist development is to incorporate β-amino acids (16, 19), but our preliminary data evaluation showed that neither PPK-AA nor other compounds containing either D-Phe or both D-Phe and β-amino acids could effectively inhibit DH activity in our diapause termination assay. In addition, the cyclic DH analog cyclo[GLWFGPRL] did not show an antagonist response in this assay (a list of compounds designed and evaluated as potential antagonists is provided in Table S1).
The results we present show that the overwintering diapause of H. zea, and presumably the diapause of other pest species, is indeed susceptible to manipulation. Through the development of rationally designed peptide agonists and an antagonist of DH, we have prevented the entry into diapause, terminated diapause prematurely, and blocked the termination of diapause in H. zea. The fact that some of these peptide agonists are much more potent than the natural hormone in preventing or breaking diapause suggests that these agents or more advanced derivatives have potential as future control agents. In addition, the fact that some of these agents can exert their effect when delivered to above-ground larvae before the onset of diapause offers possibilities for designing more biostable compounds and administering such agents to the insect at a stage when it is still accessible, before it burrows underground for entry into its overwintering diapause. Our transformation of a DH agonist into an antagonist by incorporation of a Jones trans-peptide bond mimic motif may prove a useful strategy in the development of other peptide antagonists. Using the lead compounds we have described, we anticipate developing even more potent chemical agents capable of disrupting diapause.
Materials and Methods
Insects.
Eggs of H. zea were obtained from the insectary at North Carolina State University in March 2009, and the strain has been continuously maintained in our laboratory since that time. For colony maintenance, insects of all stages were reared in a controlled-environment room at 25 °C, with a photoperiod of 14 h L/10 h D. Larvae were reared in 32-cell rearing trays and fed an artificial diet (Bio-Serv) that was replenished every 5–7 d. Adults were enclosed in a plastic cage (36 × 36 × 36 cm) covered with cheese cloth and fed a 10% (mass/vol) sucrose solution. Diapause was achieved by transferring early third-instar larvae to 18 or 21 °C, depending on the experiment, with a photoperiod of 8 h L/16 h D until pupation. In this species, migration of the eyespots is a reliable indicator of developmental progression after pupation (11). In brief, eyespots of diapausing pupae remain in the middle of the eye until diapause is terminated, whereas those of nondiapausing pupae migrate to the edge of the eye 5–7 d after pupation and finally disappear.
Peptide and Pseudopeptide Analog Synthesis and Purification.
The DH peptide used in these experiments was synthesized by Genemed Synthesis, Inc., based on the deduced amino acid sequence of DH from H. zea (20). Synthesis, purification, and characterization of the DH/pyrokinin analogs PK-2Abf (10) and 858C-1 (13) were previously described. Other DH/pyrokinin peptide analogs, including those containing β-amino acids, were synthesized using Fmoc-protected amino acids (Advanced Chemtech) on an ABI 433A automatic peptide synthesizer (Applied Biosystems) on Rink Amide resin (Novabiochem) by means of FastMoc chemistry, as described previously (21). Fmoc-β3Phe and Fmoc-β3Pro were purchased from PepTech, Inc. The purity of all peptides was assessed by analytical RP-HPLC (19, 21) and was 95% or higher. Purified peptides were characterized by MALDI-TOF-MS and amino acid analysis of hydrolysates.
Synthesis of the analog DH-Jo (Ac-GLWA[Jo]RLa) was accomplished by adapting the previously described solution phase synthesis of Jones and Ward (14) to a solid phase strategy using Rink Amide resin utilizing Fmoc-protected amino acids on an ABI 433A peptide synthesizer under previously described conditions (21). Synthesis of the backbone cyclic DH analog cyclo[Suc-RWF[dF][a4G]]RLa was carried out on Rink Amide 4-methylbenzhydrylamine resin by means of Fmoc chemistry, adopting procedures outlined previously (17, 22), and synthesis of the “head-to-tail” cyclic octapeptide DH analog cyclo[GLWFGPRL] was carried out by adopting previously described procedures (15) used for synthesis of the related cyclic pyrokinin octapeptide analog cycloLPK (cyclo[NTSFTPRL]).
Further details on purification and characterization of the compounds discussed here are presented in SI Materials and Methods. The test compounds were quantified by amino acid analysis and dissolved in 80% (vol/vol) aqueous acetonitrile with 0.1% aqueous TFA. Samples were air-dried and then dissolved in distilled water for the diapause termination and prevention assays or in 10% (vol/vol) ethanol for the DH antagonist assay.
Diapause Prevention Assay.
For this assay, H. zea was reared at 21 °C for 8 h L/16 h D from the onset of the third instar and the agents were injected into the body 24–48 h before pupation. Distilled water was used as a control. Mortality was noted 2–3 d after injection, and the pupal diapause incidence was calculated 7 d after injection.
Diapause Termination Assay.
For this assay, H. zea was reared at 18 °C for 8 h L/16 h D from the onset of the third instar onward, and fresh diet was supplied weekly. Diapause status was checked 7 d after pupation. This was the same diapause termination assay used previously (9, 10).
DH Antagonist Assay.
The ED50 for pupal diapause termination in H. zea by DH is 100 pmol per pupa, as described previously (9). Two-week-old diapausing pupae reared at 18 °C were selected for the DH antagonist assay. Two nanomoles of antagonist candidate compounds was dissolved in 10% (vol/vol) ethanol and coinjected with 100 pmol of DH into diapausing pupae. Pupae injected with 10% (vol/vol) ethanol and 100 pmol of DH dissolved in 10% (vol/vol) ethanol were used as negative and positive controls, respectively. The treated pupae were placed at 21 °C for 12 h L/12 h D, and diapause status was examined 7 d later.
Statistical Analysis.
GraphPad Prism 5 (GraphPad Software) and Origin 8.5 (OriginLab) were used for statistical analysis and data presentation. Data were subjected to a paired t test to generate two-tail P values or one-way ANOVA. A nonlinear trend line and the ED50 of DH-2Abf-K for diapause prevention were calculated by Prism 5.
Supplementary Material
Acknowledgments
We acknowledge the able technical assistance of Allison Strey and Nan Pryor (US Department of Agriculture), and we appreciate thoughtful comments on the manuscript provided by Professors Frank M. Horodyski (Ohio University) and Arnold de Loof (University of Leuven). This project was supported by US Department of Agriculture-NIFA Grant 2011-67013-30199 (to Q.Z., R.J.N., and D.L.D.) and Grant IS-4205-09C from the US-Israel Binational Agricultural Research and Development Fund (to R.J.N, K.K., and J.Z.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113863108/-/DCSupplemental.
References
- 1.Tauber MJ, Tauber CA, Masaki S. Seasonal Adaptations of Insects. New York: Oxford Univ Press; 1986. [Google Scholar]
- 2.Danks HV. Insect Dormancy: An Ecological Perspective. Ottawa: Biological Survey of Canada; 1987. [Google Scholar]
- 3.Denlinger DL, Yocum GD, Rinehart JP. In: Comprehensive Molecular Insect Science. Gilbert LI, Iatrou K, Gill SS, editors. Vol 3. Amsterdam: Elsevier; 2005. pp. 615–650. [Google Scholar]
- 4.Denlinger DL. Why study diapause? Entomol Res. 2008;38:1–9. [Google Scholar]
- 5.Wu KM, Guo YY. The evolution of cotton pest management practices in China. Annu Rev Entomol. 2005;50:31–52. doi: 10.1146/annurev.ento.50.071803.130349. [DOI] [PubMed] [Google Scholar]
- 6.Yamashita O. Diapause hormone of the silkworm, Bombyx mori: Structure, gene expression and function. J Insect Physiol. 1996;42:669–679. [Google Scholar]
- 7.Xu WH, Denlinger DL. Molecular characterization of prothoracicotropic hormone and diapause hormone in Heliothis virescens during diapause, and a new role for diapause hormone. Insect Mol Biol. 2003;12:509–516. doi: 10.1046/j.1365-2583.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhang TY, et al. The diapause hormone-pheromone biosynthesis activating neuropeptide gene of Helicoverpa armigera encodes multiple peptides that break, rather than induce, diapause. J Insect Physiol. 2004;50:547–554. doi: 10.1016/j.jinsphys.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Q, Zdarek J, Nachman RJ, Denlinger DL. Diapause hormone in the corn earworm, Helicoverpa zea: Optimum temperature for activity, structure-activity relationships, and efficacy in accelerating flesh fly pupariation. Peptides. 2008;29:196–205. doi: 10.1016/j.peptides.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Q, Nachman RJ, Zubrzak P, Denlinger DL. Conformational aspects and hyperpotent agonists of diapause hormone for termination of pupal diapause in the corn earworm. Peptides. 2009;30:596–602. doi: 10.1016/j.peptides.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Phillips JR, Newsom LD. Diapause in Heliothis zea and Heliothis virescens (Lepidoptera: Noctuidae) Ann Entomol Soc Am. 1966;59:154–159. [Google Scholar]
- 12.Zhang Q, Denlinger DL. Dynamics of diapause hormone and prothoracicotropic hormone transcript expression at diapause termination in pupae of the corn earworm, Helicoverpa zea. Peptides. 2011 doi: 10.1016/j.peptides.2011.05.019. 10.1016/j.peptides.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 13.Teal PEA, Nachman RJ. A brominated-fluorene insect neuropeptide analog exhibits pyrokinin/PBAN-specific toxicity for adult females of the tobacco budworm moth. Peptides. 2002;23:801–806. doi: 10.1016/s0196-9781(01)00656-8. [DOI] [PubMed] [Google Scholar]
- 14.Jones RCF, Ward GJ. Amide bonding isosteres: Imidazolines in pseudopeptide chemistry. Tetrahedron Lett. 1988;29:3853–3856. [Google Scholar]
- 15.Nachman RJ, Roberts VA, Dyson HJ, Holman GM, Tainer JA. Active conformation of an insect neuropeptide family. Proc Natl Acad Sci USA. 1991;88:4518–4522. doi: 10.1073/pnas.88.10.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nachman RJ. In: Biorational Control of Arthropod Pests. Ishaaya I, Horowitz AR, editors. New York: Springer; 2009. pp. 21–48. [Google Scholar]
- 17.Altstein M, et al. Backbone cyclic peptide antagonists, derived from the insect pheromone biosynthesis activating neuropeptide, inhibit sex pheromone biosynthesis in moths. J Biol Chem. 1999;274:17573–17579. doi: 10.1074/jbc.274.25.17573. [DOI] [PubMed] [Google Scholar]
- 18.Nachman RJ, et al. An amphiphilic, PK/PBAN analog is a selective pheromonotropic antagonist that penetrates the cuticle of a heliothine insect. Peptides. 2009;30:616–621. doi: 10.1016/j.peptides.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 19.Nachman RJ, et al. Biostable β-amino acid PK/PBAN analogs: Agonist and antagonist properties. Peptides. 2009;30:608–615. doi: 10.1016/j.peptides.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Ma PW, Knipple DC, Roelofs WL. Structural organization of the Helicoverpa zea gene encoding the precursor protein for pheromone biosynthesis-activating neuropeptide and other neuropeptides. Proc Natl Acad Sci USA. 1994;91:6506–6510. doi: 10.1073/pnas.91.14.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nachman RJ, Teal PE, Strey A. Enhanced oral availability/pheromonotropic activity of peptidase-resistant topical amphiphilic analogs of pyrokinin/PBAN insect neuropeptides. Peptides. 2002;23:2035–2043. doi: 10.1016/s0196-9781(02)00191-2. [DOI] [PubMed] [Google Scholar]
- 22.Zeltser I, et al. Insect neuropeptide antagonist. Part II. Synthesis and biological activity of backbone cyclic and precyclic PBAN antagonists. J Pept Res. 2001;58:275–284. doi: 10.1034/j.1399-3011.2001.00914.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.