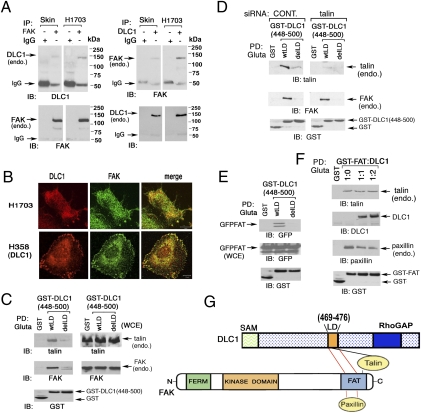
Fig. 4.
DLC1 binds FAK and interferes with paxillin binding to the FAT domain of FAK: dependence on the LD-like motif. (A) Endogenous DLC1 and FAK form a complex in human cell lines. A skin fibroblast line (Skin) and NSCLC line H1703 were analyzed by reciprocal coimmunoprecipitation. (B) Colocalization of DLC1 with FAK in NSCLC lines. Endogenous DLC1 in H1703 cells or transfected DLC1 in H358 cells were stained with anti-DLC1 antibody (red) and endogenous FAK with anti-FAK antibodies (green). The confocal images are representative of the majority of cells observed. (Scale bar: 10 μm.) (C) Association of DLC1 with talin or FAK depends on the LD-like motif. Pull down of endogenous talin and FAK by GST-fusion DLC1 fragment (448–500) with WT LD-like motif or delLD in 293T cells. (D) Interaction between DLC1 and FAK is talin-independent. Pull-down of 293T cells, with or without talin siRNA treatment, of endogenous FAK and talin by DLC1 GST (448–500) WT or delLD. (E) DLC1-FAK interaction is through the FAT-domain of FAK and requires the LD-like motif of DLC1. Pull down of GFP-FAT, derived from FAK, by GST-tagged DLC1 fragment (448–500) with WT LD-like motif or delLD in 293T cells. (F) DLC1 can compete with paxillin but not with talin for binding the FAT-domain. GST-FAT was cotransfected with increasing amounts of DLC1 at the indicated ratios in 293T cells. The pull down of DLC1, endogenous paxillin, and endogenous talin are shown, and the transfected proteins are also shown. (G) Schematic representation of the similar region in the FAT domain of FAK binds paxillin and the LD-like motif, whereas talin binds a distinct region of the FAT domain as well as the LD-like domain.