Figure 3.
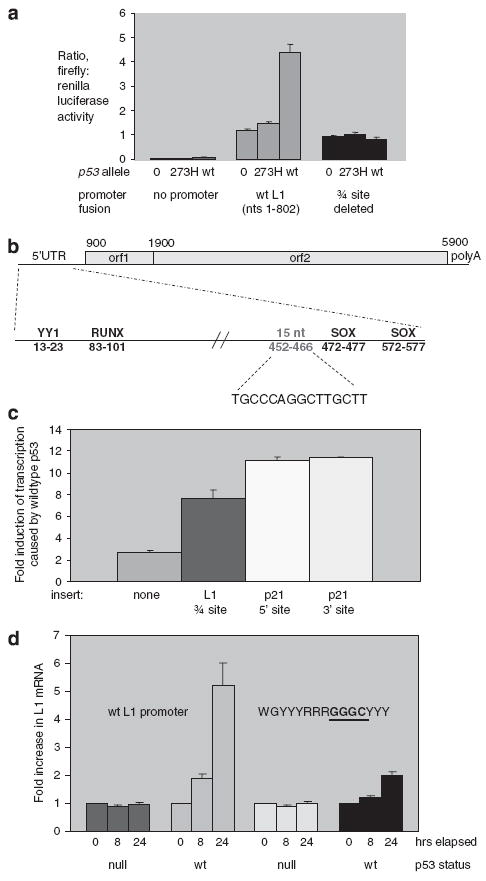
L1 (sense promoter) induction by p53, and mutational analysis. (a) Levels of transcription produced by an L1 (1–802)–firefly luciferase fusion plasmid upon cotransfection with plasmids encoding wild-type or mutant p53 were compared by luciferase assay. Twenty-four hours after cotransfection, cells were lysed and both firefly luciferase, which was expressed by the L1 promoter, and renilla luciferase, which acts as a transfection control, were assayed. Expression from L1–luciferase fusions containing mutations in the ← → ← or ← → sites were also tested. (b) Three-quarter site within the L1 promoter is shown, relative to binding site for the YY-1, RUNX and SOX transcription factor. (c) Effect of placing the three-quarter site from L1 promoter in front of a pdk4–luciferase promoter fusion. Response of the pdk4 promoter fusion to sequences added from p21 is included for comparison. (d) Levels of full-length L1 transcription produced by stable transfectants of HCT116, which has wild-type p53, in comparison with expression in HCT116 (p53–/–), which has both copies of p53 deleted. To activate p53, the cells were treated with 2 μM camptothecin, or mock-treated with dimethyl sulfoxide (DMSO) before harvesting RNA. RNA was collected after 8 and 24 h after camptothecin or DMSO treatments. The fold-increase in L1 mRNA upon camptothecin treatment is plotted on this graph. The plasmids contain either wild-type L1, or an L1 with a mutation in the site. The GGGG site is the mutant site
