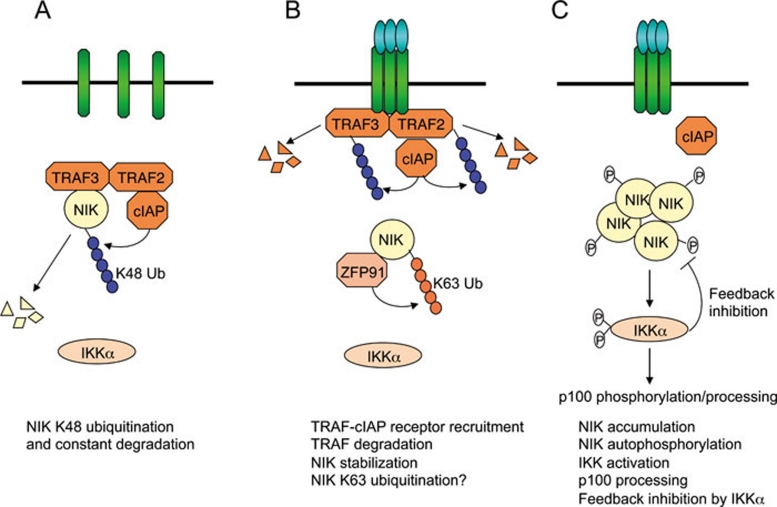
Figure 3.
NIK stabilization as a mechanism of non-canonical NF-κB signaling. (A) Under normal conditions, NIK is bound by TRAF3 and recruited to the cIAP1/2 ubiquitin ligase via TRAF3 dimerization with TRAF2. The T3-T2-cIAP E3 complex mediates constant ubiquitination and proteasomal degradation of NIK, thus preventing non-canonical NF-κB activation. (B) In response to receptor crosslinking, TRAFs and cIAP1/2 are recruited to the receptor, where cIAP1/2 ubiquitinates TRAF2 and TRAF3 and stimulates their degradation. ZFP91 mediates K63 ubiquitination of NIK, which may promote stability and catalytic activity of NIK. (C) Accumulated NIK activates IKKα, which in turn phosphorylates p100, leading to p100 processing. IKKα also phosphorylates NIK to promote NIK degradation, a feedback mechanism that may control the magnitude of NIK activation.