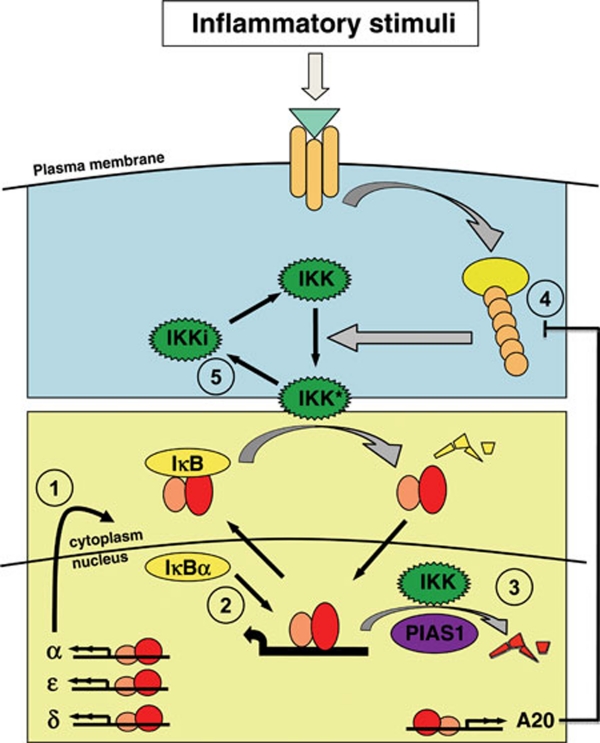
Figure 5.
Mechanisms of canonical pathway attenuation. The best characterized attenuation mechanism is negative feedback synthesis of IκB proteins (1). IκBα was recently shown to have the ability to enhance the dissociation rate of NFκB from DNA, which may facilitate its negative feedback control function (2). In addition, DNA-bound RelA NFκB, when phosphorylated on S536 by nuclear IKK complexes, was shown to be subjected to ubiquitination by the E3 ligase PIAS1, which targets NFκB to degradation (3). The ubiquitin protease A20, a highly inducible NFκB target gene, attenuates the IKK activation pathway by counteracting E3 ligases involved in the formation of ubiquitin chains that are critical signaling scaffolds (4). In addition, canonical IKK was proposed to undergo autophosphorylation that results in an inactivated kinase (5); a recycling step is necessary to return IKK back to the activatable state.