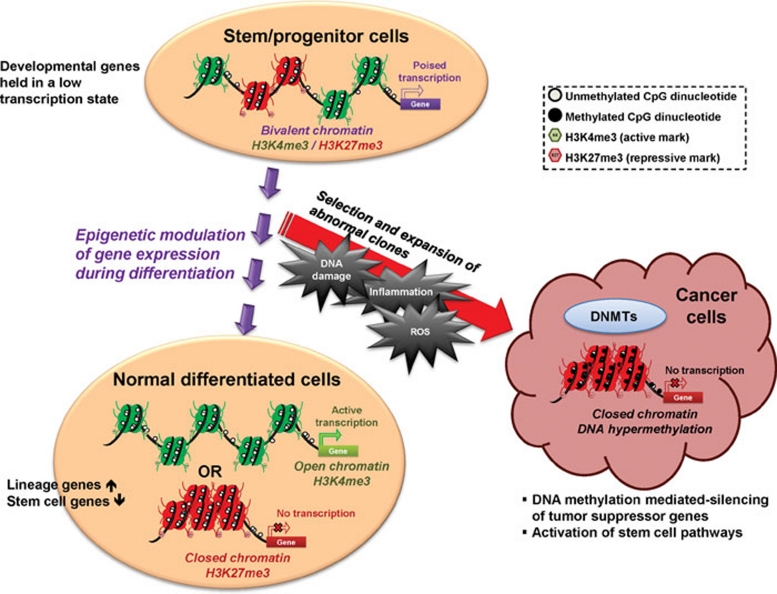
Figure 1.
In normal stem/progenitor cells, the promoter regions of many CpG island-containing developmental genes are marked by both active (trimethylated histone H3 lysine 4; H3K4me3) and repressive marks (trimethylated histone H3 lysine 27; H3K27me3), termed “bivalent chromatin” by Bernstein et al. 91. This chromatin pattern holds these genes in a low, poised transcription state to prevent premature lineage commitment. When the stem/progenitor cells respond to environmental cues and start to differentiate, a shift of the balance between the active and repressive epigenetic marks takes place with corresponding changes in chromatin architecture, leading to the silencing of stemness genes and upregulation of lineage-specific genes. However, repeated environmental stress such as chronic inflammation or accumulating reactive oxygen species (ROS) may promote clonal expansion of cells with genetic or epigenetic abnormalities, which then contribute to tumor initiation and progression. During this course of oncogenesis, the repressive marks in the promoter regions of tumor suppressor genes may recruit DNA methylation machinery to impose abnormal CpG island methylation on these genes leading to permanent gene silencing. At the same time, these epigenetic abnormalities may also contribute to activation of stem cell pathways, such as the Wnt pathway, and bestow self-renewing properties on cancer cells.