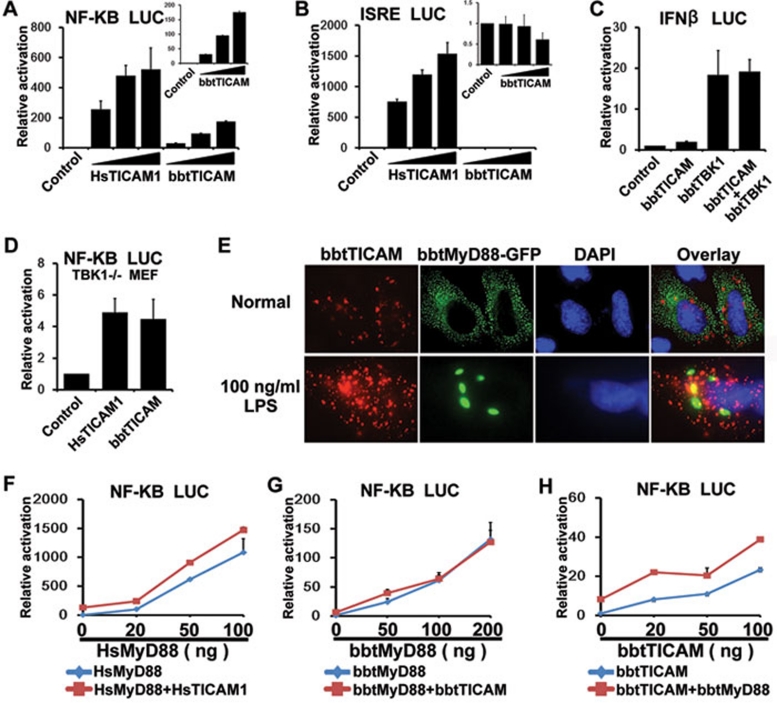
Figure 3.
bbtTICAM overexpression specifically activates a MyD88-independent NF-κB signal pathway. (A) bbtTICAM stimulates NF-κB signal pathway in a dose-dependent manner in the HEK239T cell line. (B) bbtTICAM has no effect on ISRE promoter. In A and B, HsTICAM1 (human TICAM1/TRIF) has been used as a positive control. The small panels of the bar graph show the relative activation of luciferase reporter by bbtTICAM. (C) Co-transfected HEK293T cells with bbtTICAM and bbtTBK1, IFN-β promoter was not co-stimulated. bbtTBK1 is the possible downstream molecule of bbtTICAM in amphioxus. (D) The result shows that bbtTICAM can induce the production of NF-κB as HsTICAM in TBK1−/− MEF cells. (E) bbtTICAM and bbtMyD88 have different subcellular distributions and do not co-localize in Hela cell line. At 24 h after transfection, 100 ng/ml LPS was added into the culture medium of cells for 2 h. After stimulation by LPS, the subcellular distribution of bbtTICAM is unchanged, while bbtMyD88 become larger spots and still do not co-localize with bbtTICAM. (F) HsTICAM1 and HsMyD88 (human MyD88) do not co-stimulate the signal pathway, as is the case with the controls. The HsMyD88 expression vector was titrated into transfections in HEK293T cells in the absence (blue curve) and presence (red curve) of 5 ng of the HsTICAM1 expression vector, 100 ng of the NF-κB response promoter luciferase reporter and 5 ng Rellina luciferase reporter plasmid. (G) bbtTICAM and bbtMyD88 do not co-stimulate the signal pathway. The bbtMyD88 expression vector was titrated into transfections in HEK293T cells in the absence (blue curve) and presence (red curve) of 10 ng of the bbtTICAM1 expression vector and 100 ng of the NF-κB response promoter luciferase reporter and 5 ng Rellina luciferase reporter plasmid. (H) bbtTICAM and bbtMyD88 do not co-stimulate the signal pathway. The bbtTICAM expression vector was titrated into transfections in HEK293T cells in the absence (blue curve) and presence (red curve) of 5 ng of the bbtMyD88 expression vector and 100 ng of the NF-κB response promoter luciferase reporter and 5 ng Rellina luciferase reporter plasmid. Experiments of subcellular localization were conducted in the Hela cell line. Reporter assays were done in HEK293T cell line in triplicate and repeated at least twice in all cases. Data were expressed as 'Relative activation' (mean ± sd) relative to control induction for a representative experiment.