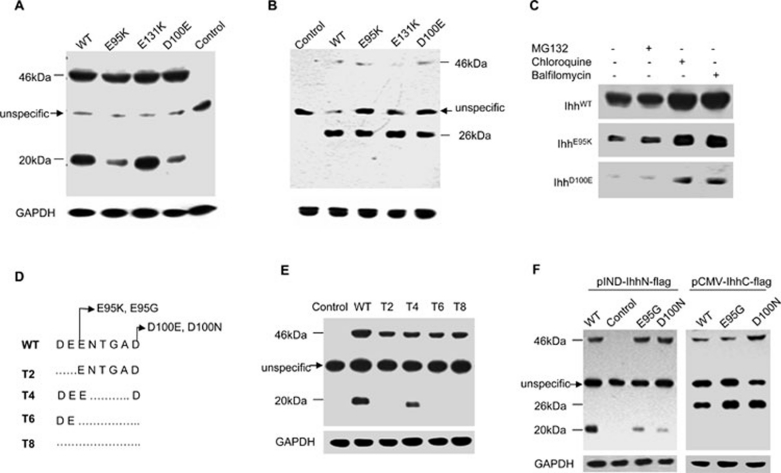
Figure 2.
Analysis of the processing and intracellular maintenance of BDA1 mutant IhhN proteins. (A, B) Cell homogenates of EcR-CHO cells transfected with the WT or mutant Ihh in which double FLAG-epitope tags were introduced into the N- (A) or C-terminus (B) of the IhhN fragment were analyzed by western blot. Molecular masses (kDa) are indicated on the left, where 46 kDa refers to the full-length IHH protein and 26 kDa and 20 kDa indicate IhhC and IhhN proteins, respectively. The arrow indicates a nonspecific band. Control refers to the cell homogenates transfected with an empty vector. Protein loading was monitored by western blotting of the mouse anti-GAPDH monoclonal antibody. The levels of E95K and D100E IhhN fragments (20 kDa) are reduced significantly (A), while the levels of IhhC fragments are comparable (B). (C) The degradation of WT and two mutant IhhN proteins is suppressed by lysosome inhibitors (chloroquine and balfilomycin), but not by a proteasome inhibitor (MG132). (D) Sketch map showing the truncation constructs. The deleted amino acids are shown as a broken line. (E) Stability of the truncated proteins in EcR-CHO cells. The IhhN fragments of T2, T6, and T8 are barely detectable, but the T4 fragment is comparable to that of WT. (F) Stability of E95G and D100N mutant proteins in EcR-CHO cells. E95G and D100N IhhN fragments are degraded, and the corresponding IhhC fragments are stable, with levels comparable to WT.