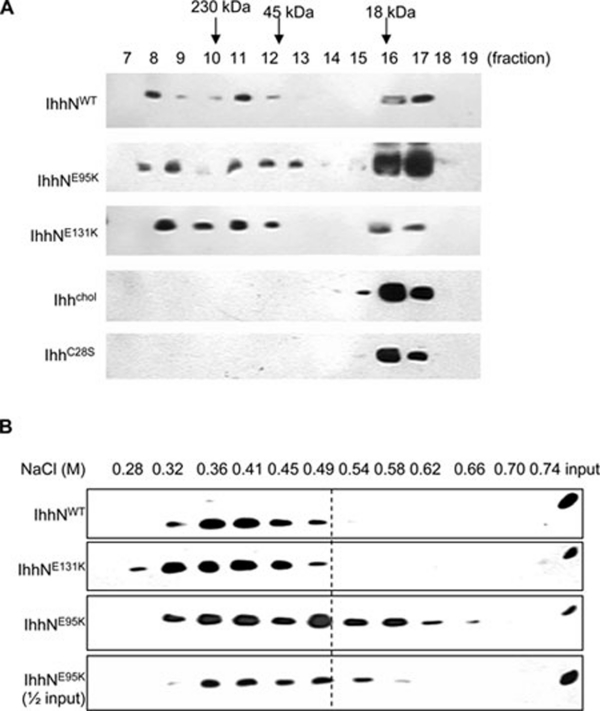
Figure 6.
Formation of multimeric complexes and heparin-binding affinity assay of WT and mutant IhhN proteins. (A) N-terminal fragment of Indian Hedgehog (IhhN) present in the conditioned medium of EcR-CHO cells was fractionated by gel-filtration chromatography to determine the presence of IHH multimers. The majority of IHH migrates as a multimer at fractions 8-12. A minor peak consistent with the monomeric form of IhhNp was observed in fractions 16 and 17. IHHChol and IHHC28S represent IHH without cholesterol and palmitate modifications, respectively. (B) The interaction between IHH and heparin by affinity chromatography. Wild type and E131K IHH showed a similar profile, eluting from the column between 0.32 and 0.49 M NaCl. However, the elution profile for E95K IhhN was extended considerably to between 0.32 and 0.66 M NaCl.