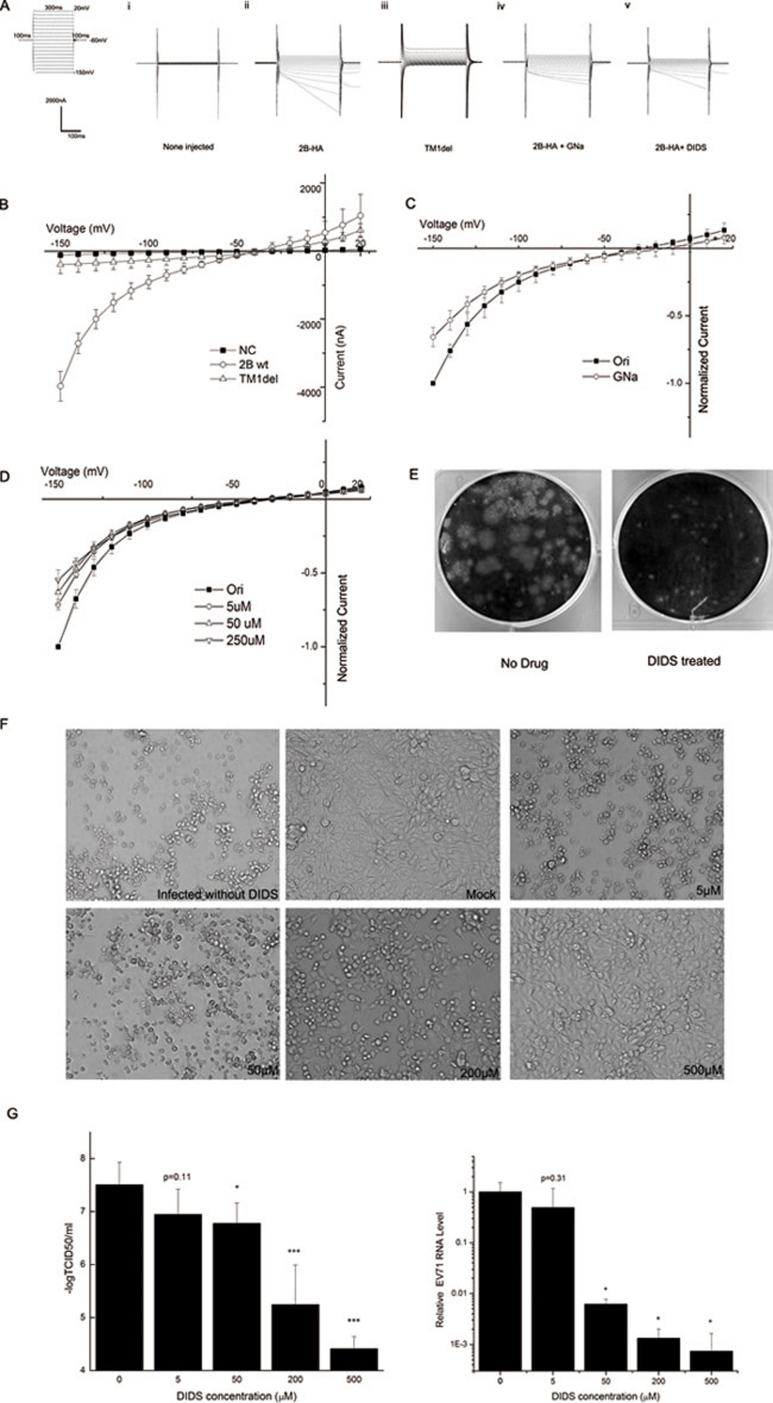
Figure 1.
(A) The general protocol used in the voltage-clamp experiments is shown in the left panel. (i, ii) At 24 to 36 h after cRNA injection, currents were recorded using a two-electrode voltage clamp, and injected and non-injected oocytes were compared. (iii) Raw current in an oocyte expressing the dysfunctional mutant TM1del. (iv) Raw current in an oocyte expressing 2B-HA after the extracellular NaCl in the oocyte Ringer solution (ORi) was replaced with equimolar sodium gluconate (GNa). (v) Raw current in an oocyte expressing 2B-HA and treated with 250 μM 4,4′-diisothiocyano-2,2′-stilbenedisulfonic acid (DIDS). (B) Current-voltage (I-V) curves of the 2B-HA-mediated currents. The normalized currents from the chloride replacement experiment are plotted in C as the current at each voltage normalized to the peak current at −150 mV. The corresponding normalized currents following the application of a given dose of DIDS are shown in D. The data in B-D are presented as mean ± SD and were generated from at least three separate experiments. (E) A plaque assay was performed in cells treated with or without 10 μM DIDS. (F) RD cells were mock infected or infected with EV71 at an MOI of 0.002 and then cultured in medium containing the indicated concentration of DIDS for an additional 48 h. The cytopathic effect (CPE) was observed using microscopy (at 100× magnification). (G) RD cells were infected with the EV71 virus at an MOI of 0.002 for 30 min, and cultured in fresh medium containing indicated concentration of inhibitor. Two days after infection, the supernatant was collected for tissue culture infective dose (TCID50) analysis (left panel) or real-time RT-PCR analysis (right panel). The data are presented as mean ± SD and were generated from at least three separate experiments. (*P < 0.05, ***P < 0.001, compared with the no DIDS group)