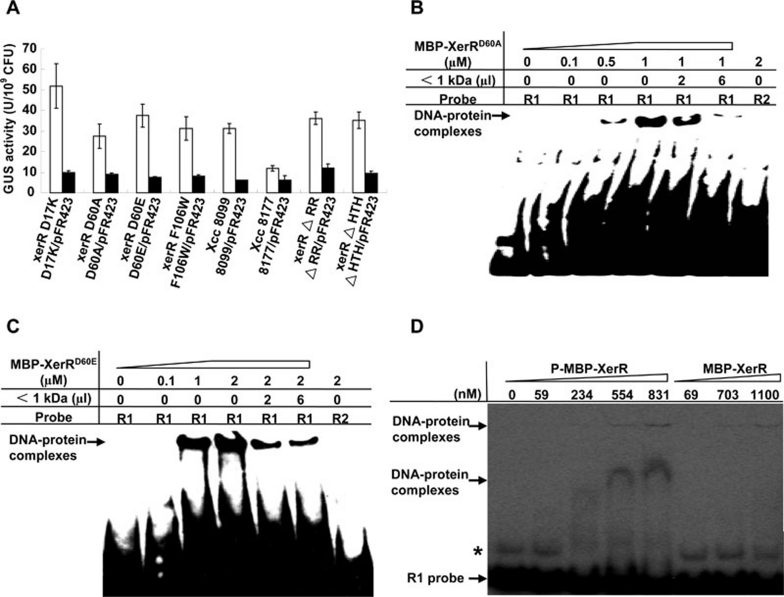
Figure 3.
Phosphorylation-related residues of XerR are essential for its repressor function. (A) The conserved phosphorylation-related residues and the regulatory domains of XerR were indispensable for regulation of xccR expression in vivo. xerR ΔRR, xerR ΔHTH and different site-directed mutants exhibited increased GUS activities when grown in NYG medium. Plasmid-containing (pFR423) strains of different mutants reduced the GUS activities compared with that of Xcc 8177. Bacteria cultured in NYG medium were assayed at an OD600 of 2.0. The experiments were repeated eight times with similar results. (B and C) EMSA assays of MBP-XerRD60A and MBP-XerRD60E with biotin-labeled R1 probe and plant extract. The two proteins presented the same binding characteristics to R1 probe, in which the plant extract of molecular weights < 1 kDa released the protein and DNA interactions. In the diagram, the concentration of purified protein and the volumes of plant signal(s) are indicated. (D) EMSA binding of phosphorylated and unphosphorylated MBP-XerR to the R1 probe. MBP-XerR was phosphorylated in vitro with acetyl phosphate and the R1 probe was end-labeled with 32P at its 5′ termini. The bands marked with an asterisk indicate a possible higher structure of R1 probe formed during annealing step.