Abstract
Objective
The aim of this study was to evaluate the clinical characteristics and survival outcomes of patients with primary mediastinal germ cell tumor (PMGCT) by identifying the prognostic factors and efficacies of different treatment modalities.
Methods
Fifty-five patients with PMGCT who were treated consecutively at Cancer Center, Sun Yat-sen University, Guangzhou, from 1988 to 2010 were evaluated retrospectively.
Results
Fifty-two men and 3 women with a median age of 25 years were identified, of whom 17 (30.9%) had pure seminomatous, 38 (69.1%) had nonseminomatous histology, 27 (49.1%) had tumor located at mediastinum, 20 (36.4%) had lung metastases and/or effusions, and 8 (14.5%) had distant metastases. Three treatments surgery, chemotherapy, and radiotherapy were performed in 11 (20%) patients, two treatments chemotherapy plus surgery or radiotherapy were performed in 25 (45.6%), and single treatment surgery or chemotherapy was performed in 17 (30.9%). The other two patients (3.6%) received no treatment. After a median follow-up time of 31.4 months, the 5-year survival rate was 52%. The median overall survival time was 87.9 months. Patients who received two treatments had the longest survival time of 118.3 months, P = 0.000. Those who had pure seminoma histology, whose tumor confined to the mediastinum and who achieved complete or partial remission at initial evaluation, who had complete resection and radiotherapy were considered to have better prognosis according to univariate analysis. On multivariate analysis, extension and response rate at initial evaluation were independently predictive of survival.
Conclusions
Primary mediastinal germ cell tumor is rare with a dominant frequency in young male patients. Chemotherapy combined with local therapy like surgery or radiotherapy is a reasonable treatment strategy recommended. Extension and initial remission rate are independent prognostic factors.
Keywords: Primary mediastinal germ cell tumor, Treatment strategies, Prognostic factors, Extension, Response rate
Introduction
Primary mediastinal germ cell tumor is a rare subgroup of germ cell tumors reported to account for less than 5% of germ cell malignancies. It has similar clinical characteristics and histopathologic distributions as its counterparts in gonads (Moran et al. 1997a, b), though diverse features exist. Literatures reported that it is more common in men than in women. In addition, it displays different biological behaviors in men than those originate in testis or in women (Rodney et al. 2010, Schneider et al. 2004).
Histologically, it can be classically divided into two categories: seminomas and nonseminomatous. Each histological subtype can be associated with another subtype that realizes a so-called mixed germ cell tumor (Chetaille et al. 2010).
Patients with pure seminomatous histology in the mediastinum were reported to have chances of long-term cure of almost 90%, but only 45% of patients with mediastinal nonseminomas were estimated to be alive at 5 years, of whom outcomes were obviously worse than those in gonads or retroperitoneal primary (Bokemeyer et al. 2002).
The most important attention is about the therapeutic strategies. It was reported that cisplatin-based chemotherapy regimens given to a maximum effect followed by surgical consolidation resulted in long-term progression-free survival and overall survival time, which was recommended as the first-line therapy. At relapse, earlier administration of second-line chemotherapy established with a basis on prognostic factors including staging system or tumor markers status and so on may improve survival and quality of life showed in several studies. High-dose chemotherapy supported by stem cell transplantation was also attempted in patients with relapsed or refractory germ cell tumor or who obtained partial remission after first-line treatment by researchers who indicated that an effective mean can be reached (Rodney et al. 2010; Schneider et al. 2000; Kang et al. 2008; Rick et al. 2004; Ishioka et al. 2010; Hara et al. 2006).
In literatures, histology (Takeda et al. 2003), extended metastases, the number of metastatic sites (Bokemeyer et al. 2001), surgical resection of the tumor, pathological evidence of persistent viable tumor in resected remnants (Rivera et al. 2010), elevated serum tumor markers after operation (Kesler et al. 2008), and so on were considered to be independent prognostic factors.
Owing to the rarity of the type of tumor, in recent 20 years, institutional publications on large numbers of patients with primary mediastinal germ cell tumors are rare, many of which are retrospective. No consensus of the treatment strategies has been reached already. In order to get more information of this type of tumor, with the ultimate aim of improving the treatment effect, we retrospectively analyzed the patients with primary mediastinal germ cell tumor visited at our hospital in recent years regarding the clinicopathological characteristics and survival outcomes.
Materials and methods
Patients
From 1988 to 2010, 55 patients with primary mediastinal germ cell tumor treated at Cancer Center, Sun Yat-sen University, Guangzhou, were identified, whose clinicopathological characteristics including age, sex, histology, disease extent, tumor markers before treatment, treatment scenarios, and initial response rate were retrospectively studied. Follow-ups were done through telephone connection or outpatient meeting in order to confirm the survival status of the patients. Fifty-two patients were referred at the initial diagnosis. The other three patients were referred after the surgical resection of the tumor elsewhere. Bulky masses located at the mediastinum presented in all patients as chest X-ray or CT scan showed before treatment. No clinical or imaging detectable testicular or ovarian masses existed. Histological diagnosis was made through core needle biopsy or fine-needle aspiration plus serum tumor markers alpha-fetoprotein (AFP) or human chorionic gonadotropin (beta-HCG) before therapy.
Treatment strategies
Triple-modality therapy including surgery followed by chemotherapy and radiotherapy was performed in six patients, and chemotherapy followed by surgery and radiotherapy was performed in five patients. Twelve patients received chemotherapy plus radiotherapy. Seven patients received surgery followed by chemotherapy. Five patients received chemotherapy followed by surgery. Chemotherapy alone was offered to 16 patients. Two patients underwent surgery alone. Two patients did not receive any treatment owing to the poor performance status. In 51 patients who have received chemotherapy, one to 14 courses of chemotherapy given at 3-week intervals were provided, with the mean of 4.92 courses.
Initial chemotherapy regimens were included as follows: doxorubicin plus vincristine plus bleomycin in two patients, cyclophosphamide plus cisplatin plus doxorubicin in five patients, cyclophosphamide plus etoposide plus cisplatin in four patients, bleomycin plus etoposide plus cisplatin in 30 patients, and etoposide plus ifosfamide plus cisplatin in ten patients.
Response criteria
Complete resection was defined as no macroscopic or microscopic residual tumor. Incomplete resection was considered that evident macroscopic or microscopic residual tumor existed. Initial evaluation was defined as the response rate evaluated after initial treatment including surgery or two courses of chemotherapy. A complete response (CR) was defined as clinical or radiographic disappearance of tumors and normalization of AFP and beta-HCG. A partial response (PR) was defined as residual imaging abnormalities with more than 50% decrease in either the primary or metastatic sites and/or declining markers. Patients with more than 25% increase in tumor size, new lesions or increasing markers were considered as having progressive disease (PD). Stable disease (SD) was defined as neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD.
Statistical analysis
Follow-up time was defined from the date of pathological diagnosis to the date of death or last contact. A chi-square test was used to compare the proportions of different groups. Survival time was estimated using life table method and Kaplan–Meier method. The univariate influence of each potential prognostic factor on overall survival was analyzed by the log-rank test. Cox proportional hazard model was used as multivariate analysis to investigate the factors that had independent prognostic value. All data analyses were performed using SPSS version 16.0. A P < 0.05 was regarded as statistically significant.
Results
Clinical findings
Primary mediastinal germ cell tumors occurred predominantly in young adults with a mean age of 24.65 years (range, 12–64). Fifty-two men (94.5%) and 3 women (5.5%) were identified. The age spectrum was different between men and women, with a mean age of 23.67 years in men and 41.67 years in women. Seventeen (30.9%) patients were diagnosed as having pure seminoma and 38 (69.1%) had nonseminomatous including embryonal carcinoma, yolk-sac tumor, teratoma, and mixed nonseminomas. Twenty-seven (49.1%) patients had tumors located at the mediastinum, 20 (36.4%) had lung metastases and/or plural or pericardial effusion, and 8 (14.5%) had tumors metastasized to distant sites.
At the time of diagnosis, 46 (83.6%) patients complained of nonspecific symptoms, such as cough, chest pain, dyspnea, low fever, and apnea. Of 46 patients, 8 (14.5%) had superior vena caval syndrome and 9 (16.4%) other patients were asymptomatic whose tumors were found through routine health examination. A majority of patients were presented as enormous masses in the mediastinum encroaching or compressing adjacent organs such as bronchus, heart, and great vessels. Two patients had poor performance status leading to the unavailability of treatment. The performance status of others ranges from 0 to 2 (Table 1).
Table 1.
Characteristics of 55 patients with primary mediastinal germ cell tumor
| Characteristics | No. of patients | % |
|---|---|---|
| Age | ||
| ≤15 years | 5 | 9.1 |
| >15 years | 50 | 90.9 |
| Sex | ||
| Male | 52 | 94.5 |
| Female | 3 | 4.5 |
| Histopathology | ||
| Pure seminomatous | 17 | 30.9 |
| Teratoma | 13 | 23.6 |
| Embryonal | 4 | 7.3 |
| Yolk sac | 2 | 3.6 |
| Mixed nonseminomatous | 18 | 32.7 |
| Symptoms | ||
| Symptomatic | 46 | 83.6 |
| Asymptomatic | 9 | 16.4 |
| Superior vena caval syndrome | 8 | 14.5 |
| Disease extent | ||
| Circumscribed | 27 | 49.1 |
| Metastasizing to lung and/or effusions | 20 | 36.4 |
| Metastasizing to distant places | 8 | 14.5 |
| Chemotherapy courses in 51 patients | ||
| 1–4 courses | 25 | 49 |
| More than 4 courses | 26 | 51 |
Treatment outcomes
Complete resection was available in 22 patients, whereas incomplete resection was performed in seven patients, including biopsy in four and hemisection in two. There was no discrimination of complete resectability between patients who had surgery as initial treatment (13 in 19) or post-chemotherapy (9 in 10) (P = 0.367). Viable cells in the post-excisional specimens were found in ten patients after complete resection. The toxicities of chemotherapy were tolerated. At initial evaluation, 13 (23.6%) patients achieved CR, 18 (32.7%) achieved PR, 9 (16.4%) developed PD, and 13 (23.6%) remained stable.
Survival
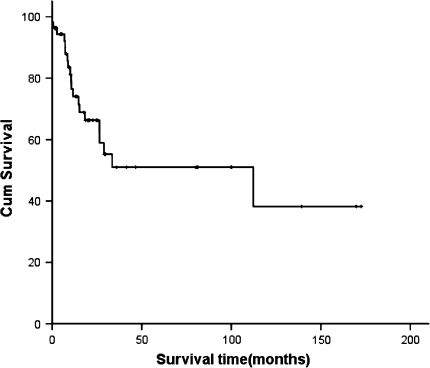
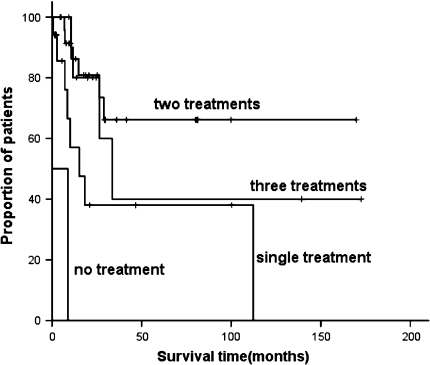
With a median follow-up of 31.4 months (0.43–172.6 months), the 5-year survival time of the whole patient group was 52%. The median overall survival time was 87.9 months. Patients who received two treatments had the longest survival time of 118.3 months compared with those received three (83.26 months), one (48.63 months) or no treatment (4.65 months), P = 0.000. Five-year survival rate of patients who had pure seminomatous histology was statistically better than that of those with nonseminomatous (87% vs. 33%, P = 0.018). When categorized patients in terms of disease extent, that is localized at the mediastinum, metastasized to lungs and/or effusions, or had metastases to distant sites, differences in survival existed, 72%, 45%, zero of three groups, respectively, P = 0.005. Furthermore, patients who achieved CR or PR at initial evaluation (59, 70, 45, 0% for patients obtained CR, PR, SD, PD, respectively, P = 0.000), or had neoplasms completely resected (58, 29, 52% for completely resected, incompletely resected, no resection, respectively, P = 0.028), and received radiotherapy (60% vs. 43%, P = 0.008) were considered to have better prognosis according to univariate analysis (Table 2; Figs. 1, 2).
Table 2.
The median overall survival time of patients with primary mediastinal germ cell tumor according to different characteristics
| No. of patients | OS (months) | P | |
|---|---|---|---|
| Sex | 0.738 | ||
| Males | 52 | 92.04 | |
| Females | 3 | 112.37 | |
| Histopathology | 0.006 | ||
| Pure seminomatous | 17 | 148.99 | |
| Nonseminomatous | 38 | 57.69 | |
| Extent of disease | 0.002 | ||
| Circumscribed | 27 | 113.90 | |
| Lung metastases and/or effusions | 20 | 69.53 | |
| Distant metastases | 8 | 14.33 | |
| Treatment scenarios | 0.000 | ||
| Three treatments | 11 | 83.26 | |
| Two treatments | 25 | 118.27 | |
| One treatments | 17 | 48.63 | |
| No treatments | 2 | 4.65 | |
| Resection | 0.031 | ||
| Complete | 22 | 96.23 | |
| Incomplete | 7 | 55.36 | |
| No resection | 26 | 48.20 | |
| Radiotherapy | 0.026 | ||
| With | 23 | 111.04 | |
| Without | 32 | 66.56 | |
| Initial evaluation | 0.000 | ||
| CR + PR | 31 | 106.46 | |
| SD | 13 | 46.00 | |
| PD | 11 | 10.87 | |
Fig. 1.
The survival curve of 55 patients with primary mediastinal germ cell tumors
Fig. 2.
The survival curves of patients with primary mediastinal germ cell tumors with different treatment strategies
Multivariate analysis
Potential prognostic factors were included in regression models if there was statistical evidence of, or a theoretical basis for, a relationship to overall survival. Two significant predictors of poor overall survival were identified: extensive extent (P = 0.012) and poor response rate at initial evaluation (P = 0.002). These factors were independently predictive of lower probability of overall survival. The association between overall survival and gender (P = 0.657), age at diagnosis (P = 0.59), histology (P = 0.119), chemotherapy courses (P = 0.248), and resectability (P = 0.59) could not be established (Table 3).
Table 3.
The outcomes of multivariate analysis by Cox proportional hazard model 95% CI for Exp(B)
| B | SE | Wald | df | Sig. | Exp(B) | Lower | Upper | |
|---|---|---|---|---|---|---|---|---|
| Tumor extension | 1.087 | 0.436 | 6.223 | 1 | 0.013 | 2.964 | 1.262 | 6.960 |
| Initial remission rate | 0.838 | 0.280 | 8.939 | 1 | 0.003 | 2.311 | 1.335 | 4.003 |
Discussion
In this retrospective analysis of patients with primary mediastinal germ cell tumors, a predominant frequency of morbidity in young adult patients was identified. The mean age of the whole group was 24.65 years, of whom 50 were older than 15 years. The peak of incidence was greater than their counterparts in gonads contrasted historically. For example, Dominik et al. reported 1,157 patients with germ cell tumors in gonads of 1,442 patients were younger than 15 years in an epidemiological analysis (Schneider et al. 2004). Only 3 female patients with a mean age of 41.67 years were identified (sex ratio 17.3). This gender and age tendencies were also reported by other studies (Rivera et al. 2010; Nakamura et al. 2009).
At diagnosis, many patients complained of nonspecific symptoms resembling common respiratory diseases, including cough, chest pain, dyspnea, low fever, and apnea. In addition, some patients were asymptomatic. It is one of the causes leading to the postponing of diagnosis and treatments. In the present study, more than half of the patients have had tumors metastasized to lungs or distant sites already at the time of visiting doctors. A statistically significant poorer survival of patients with extensive diseases compared to those circumscribed was identified. Five-year overall survival rate of patients who had tumors localized at the mediastinum, metastasized to lungs or distant sites were 72%, 45%, and 0, respectively, P = 0.005. We obtained an opinion which was agreed by previous experts that earlier diagnosis and treatments are key strategies to improving the therapeutic efficacies and overall survival of patients with primary mediastinal germ cell tumors (Moran et al. 1997c).
Imaging tests like chest X-ray and CT scan can be used to displaying tumor lesions. A biopsy through fine-needle aspiration or mediastinoscopy plus tumor markers AFP and beta-HCG are methods helping determine the histopathological diagnosis. Gilligan et al. made a meta-analysis in which serum tumor markers AFP or beta-HCG or lactate dehydrogenase are recommended to be used to stage patients or monitor for relapse in patients with nonseminomatous or advanced seminoma only, not to screen or decide management strategies (Gilligan et al. 2010), we could not make a definite recommendation for the use of biomarkers owing to the deficit of data.
Histology is an important prognostic factor indicating the survival outcome of patients as elucidated by many literatures. In our study, we also found an evident discrimination of survival between patients with pure seminomatous and nonseminomatous (5-year survival rate, 87 and 33%, respectively, P = 0.018) despite no statistically significant differences according to the multivariate analysis.
With regard to the therapeutic strategies, previous experts summarized their experiences including surgery, chemotherapy, radiotherapy, and high-dose chemotherapy with stem cell support, using small samples (Rodney et al. 2010; Kang et al. 2008; Bokemeyer et al. 2001; Lopes et al. 2009; Sakurai et al. 2004; Bokemeyer et al. 2003; Mann et al. 2000). In this study, patients were categorized into four groups: those who received three treatments surgery, chemotherapy, and radiotherapy irrespective of the sequence, those who received two treatments chemotherapy plus surgery or radiotherapy, and those who received single treatment chemotherapy or surgery. Another two patients received no therapy. According to the result of log-rank test, the patients who received two treatments had the longest survival time of 118.3 months. There were several reasons. The superior and lasting toxicities of three treatments are attributed to the worse survival, whereas single treatment is incapable of eliminating the residual tumor or micrometastases after chemotherapy or surgery due to the extensive properties of germ cell tumors in the mediastinum. Two treatments chemotherapy plus local therapy surgery or radiotherapy may be reasonable management strategies recommended. But more explorations are required to confirm this.
Patients with primary mediastinal germ cell tumors were classified as poor prognosis group according to IGCCC (Mead and Stenning 1997). Several prognostic factors were identified to better tailoring treatment. For example, Bokemeyer et al. evaluated 635 patients with extragonadal germ cell tumor over 21 years at 11 centers and concluded that liver metastases and two or more metastatic sites influenced survival negatively (Bokemeyer et al. 2001). In this study, according to multivariate analysis, disease extension and response rate at initial evaluation were independent prognostic factors. We recommended that patients who did not reach a complete or partial remission at initial evaluation should be considered for more intensive therapies if tolerable.
However, due to the retrospective property and small sample size of this study, much still remains to be done in order to improving the survival of patients with PMGCT.
Conflict of interest
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Bokemeyer C, Droz JP, Horwich A, et al. Extragonadal seminoma: an international multicenter analysis of prognostic factors and long term treatment outcome. Cancer. 2001;91:1394–1401. doi: 10.1002/1097-0142(20010401)91:7<1394::AID-CNCR1144>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Bokemeyer C, Nichols CR, Droz JP, et al. Extragonadal germ cell tumors of the mediastinum and retroperitoneum: results from an international analysis. J Clin Oncol. 2002;20:1864–1873. doi: 10.1200/JCO.2002.07.062. [DOI] [PubMed] [Google Scholar]
- Bokemeyer C, Schleucher N, Metzner B, et al. First-line sequential high-dose VIP chemotherapy with autologous transplantation for patients with primary mediastinal nonseminomatous germ cell tumours: a prospective trial. Br J Cancer. 2003;89:29–35. doi: 10.1038/sj.bjc.6600999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetaille B, Massard G, Falcoz PE, et al. Mediastinal germ cell tumors: anatomopathology, classification, teratomas and malignant tumors. Rev Pneumol Clin. 2010;66:63–70. doi: 10.1016/j.pneumo.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Gilligan TD, Seidenfeld J, Basch EM, et al. American Society of Clinical Oncology Clinical Practice Guideline on uses of serum tumor markers in adult males with germ cell tumors. J Clin Oncol. 2010;28:3388–3404. doi: 10.1200/JCO.2009.26.4481. [DOI] [PubMed] [Google Scholar]
- Hara I, Miyake H, Yamada Y, et al. Feasibility and usefulness of high-dose chemotherapy (high-dose ifosfamide, carboplatin and etoposide) combined with peripheral blood stem cell transplantation for male germ cell tumor: a single-institute experience. Anticancer Drugs. 2006;17:1057–1066. doi: 10.1097/01.cad.0000231469.46664.12. [DOI] [PubMed] [Google Scholar]
- Ishioka J, Kageyama Y, Inoue M, et al. Result of treatment for advanced germ cell tumor in the last decade. Nippon Hinyokika Gakkai Zasshi. 2010;101:539–546. doi: 10.5980/jpnjurol.101.539. [DOI] [PubMed] [Google Scholar]
- Kang CH, Kim YT, Jheon SH, et al. Surgical treatment of malignant mediastinal nonseminomatous germ cell tumor. Ann Thorac Surg. 2008;85:379–384. doi: 10.1016/j.athoracsur.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Kesler KA, Rieger KM, Hammoud ZT, et al. A 25-year single institution experience with surgery for primary mediastinal nonseminomatous germ cell tumors. Ann Thorac Surg. 2008;85:371–378. doi: 10.1016/j.athoracsur.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Lopes LF, Macedo CR, Pontes EM, et al. Cisplatin and etoposide in childhood germ cell tumor: Brazilian pediatric oncology society protocol GCT-91. J Clin Oncol. 2009;27:1297–1303. doi: 10.1200/JCO.2008.16.4202. [DOI] [PubMed] [Google Scholar]
- Mann JR, Raafat F, Robinson K, et al. The United Kingdom Children’s Cancer Study Group’s second germ cell tumor study: carboplatin, etoposide, and bleomycin are effective treatment for children with malignant extracranial germ cell tumors, with acceptable toxicity. J Clin Oncol. 2000;18:3809–3818. doi: 10.1200/JCO.2000.18.22.3809. [DOI] [PubMed] [Google Scholar]
- Mead GM, Stenning SP. The International Germ Cell Consensus Classification: a new prognostic factor-based staging classification for metastatic germ cell tumours. Clin Oncol (R Coll Radiol) 1997;9:207–209. doi: 10.1016/S0936-6555(97)80001-5. [DOI] [PubMed] [Google Scholar]
- Moran CA, Suster S, et al. Primary germ cell tumors of the mediastinum: I. Analysis of 322 cases with special emphasis on teratomatous lesions and a proposal for histopathologic classification and clinical staging. Cancer. 1997;80:681–690. doi: 10.1002/(SICI)1097-0142(19970815)80:4<681::AID-CNCR6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Moran CA, Suster S, Koss MN, et al. Primary germ cell tumors of the mediastinum: III. Yolk sac tumor, embryonal carcinoma, choriocarcinoma, and combined nonteratomatous germ cell tumors of the mediastinum—a clinicopathologic and immunohistochemical study of 64 cases. Cancer. 1997;80:699–707. doi: 10.1002/(SICI)1097-0142(19970815)80:4<699::AID-CNCR8>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Moran CA, Suster S, Przygodzki RM, et al. Primary germ cell tumors of the mediastinum: II. Mediastinal seminomas—a clinicopathologic and immunohistochemical study of 120 cases. Cancer. 1997;80:691–698. doi: 10.1002/(SICI)1097-0142(19970815)80:4<691::AID-CNCR7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Matsumura A, Katsura H, et al. Cisplatin-based chemotherapy followed by surgery for malignant nonseminomatous germ cell tumor of mediastinum: one institution’s experience. Gen Thorac Cardiovasc Surg. 2009;57:363–368. doi: 10.1007/s11748-008-0375-z. [DOI] [PubMed] [Google Scholar]
- Rick O, Bokemeyer C, Weinknecht S, et al. Residual tumor resection after high-dose chemotherapy in patients with relapsed or refractory germ cell cancer. J Clin Oncol. 2004;22:3713–3719. doi: 10.1200/JCO.2004.07.124. [DOI] [PubMed] [Google Scholar]
- Rivera C, Arame A, Jougon J, et al. Prognostic factors in patients with primary mediastinal germ cell tumors, a surgical multicenter retrospective study. Interact Cardiovasc Thorac Surg. 2010;11:585–589. doi: 10.1510/icvts.2010.238717. [DOI] [PubMed] [Google Scholar]
- Rodney AJ, Tannir NM, Siefker-Radtke AO, et al. Survival outcomes for men with mediastinal germ-cell tumors: the University of Texas M. Anderson Cancer Center experience Urol Oncol: D; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai H, Asamura H, Suzuki K, et al. Management of primary malignant germ cell tumor of the mediastinum. Jpn J Clin Oncol. 2004;34:386–392. doi: 10.1093/jjco/hyh062. [DOI] [PubMed] [Google Scholar]
- Schneider DT, Calaminus G, Reinhard H, et al. Primary mediastinal germ cell tumors in children and adolescents: results of the German cooperative protocols MAKEI 83/86, 89, and 96. J Clin Oncol. 2000;18:832–839. doi: 10.1200/JCO.2000.18.4.832. [DOI] [PubMed] [Google Scholar]
- Schneider DT, Calaminus G, Koch S, et al. Epidemiologic analysis of 1, 442 children and adolescents registered in the German germ cell tumor protocols. Pediatr Blood Cancer. 2004;2:169–175. doi: 10.1002/pbc.10321. [DOI] [PubMed] [Google Scholar]
- Takeda S, Miyoshi S, Ohta M, et al. Primary germ cell tumors in the mediastinum: a 50-year experience at a single Japanese institution. Cancer. 2003;97:367–376. doi: 10.1002/cncr.11068. [DOI] [PubMed] [Google Scholar]