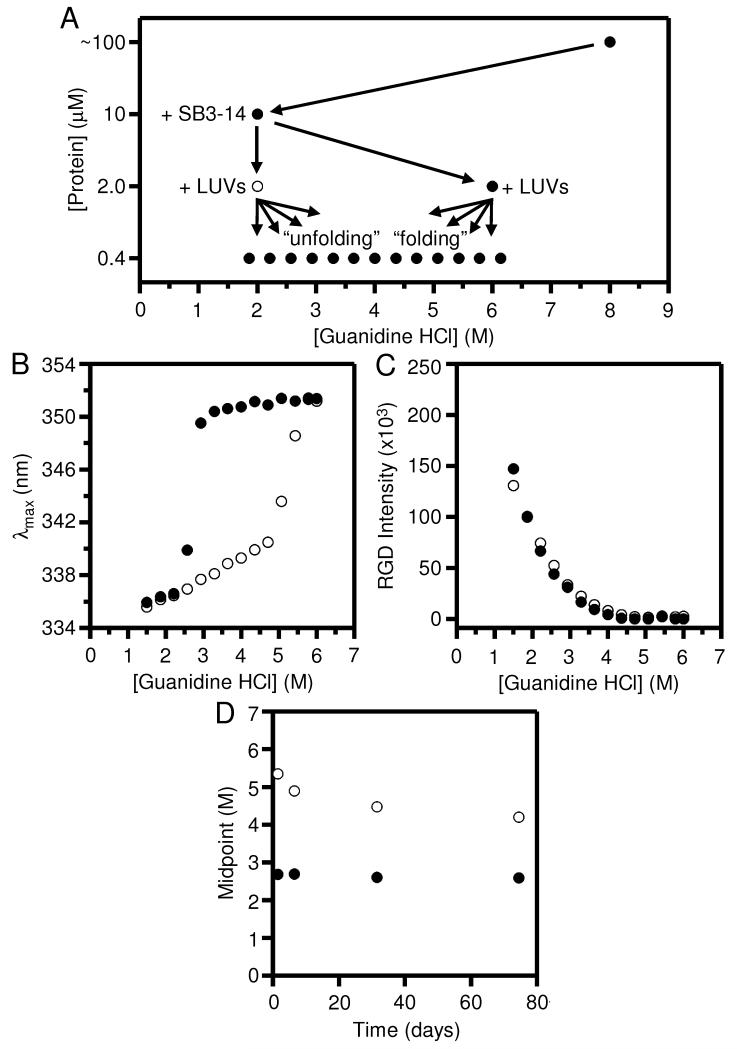
Fig. 2.
Folding and unfolding of OmpLA remain irreversible at pH 8.0, even when SB3-14 is included as a holdase to prevent OmpLA from aggregating. The excitation wavelength for all protein fluorescence samples was 295 nm. (A) General dilution scheme for the titrations that included the SB3-14. (B) Wavelength position of maximum fluorescence intensity (λmax) for samples of OmpLA in folding (●) and unfolding (○) titrations with LUVs of DLPC at 37° C and at pH 8.0 after 40 hours. The titrations were prepared according to the scheme in (A). (C) Rayleigh-Gans-Debye (RGD) light scattering at 295 nm for the same folding and unfolding titrations shown in (B). (D) Concentration of guanidine HCl where the λmax was at its midpoint of the transition between folded and unfolded protein for the same titrations in (B) measured at various time points.