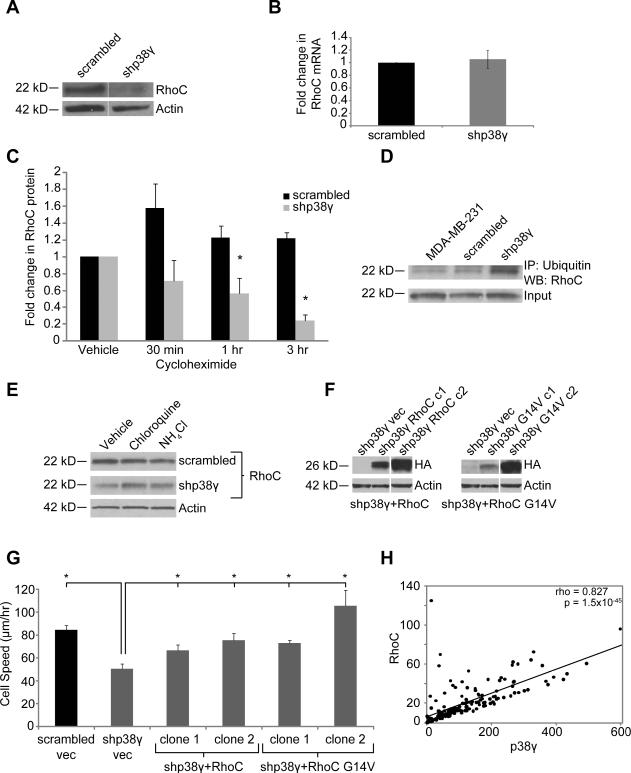
Figure 3. p38γ affects RhoC expression by mediating RhoC ubiquitination and lysosomal degradation.
Expression of RhoC GTPase protein (A) but not mRNA (B) is significantly reduced by p38γ knockdown. (C) Cycloheximide treatment reveals that RhoC protein is less stable in shp38γ cells. (D) RhoC ubiquitination is increased in shp38γ cells. (E) RhoC is degraded by the lysosome, as treatment with lysosome inhibitors leads to an increase in RhoC protein in shp38γ cells. (F) shp38γ cells were stably transfected with either HA-tagged RhoC (left) or a constitutively active form of RhoC, RhoC G14V (right). Two clones—one high expressing and one low expressing—were selected for each construct. (G) Re-expressing RhoC in shp38γ cells significantly increases shp38γ cell speed to levels comparable to scrambled cells (*p<0.05). (H) Expression of p38γ and RhoC are strongly positively correlated in clinical BC samples (n=177, rho = 0.827, p=1.5 × 10−45).