Table 2.
Double-Michael Annulations of Various Dinucleophilesa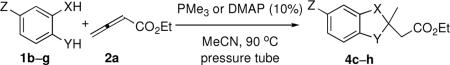
| entry | X,Y | Z | product | yield (%)b | |
|---|---|---|---|---|---|
| PMe3 | DMAP | ||||
| 1 | O, S (1b) | H | 4c | 93 | |
| 2 | O, O (1c) | H | 4d | 80 | |
| 3 | S, S (1d) | H | 4e | 74 | |
| 4c | S,NTs (1e) | H | 4f | 68 | 53 |
| 5c | NTs, NTs (1f) | H | 4g | 79 | 38 |
| 6 | O, NTs (1g) | CI | 4h | 84 | |
Reactions were performed using 0.4 mmol of 1 and 1.1 equiv of 2a.
Isolated yield after chromatography.
Reaction performed initially at 90 °C to obtain the mono-Michael adduct; the temperature was then raised to 120 °C for full conversation to the double-Michael product.
