Table 3.
Double-Michael Annulations of Substituted Allenoatesa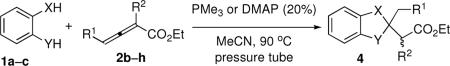
| entry | X, Y | R1,R2 | product | yield (%)b | |
|---|---|---|---|---|---|
| PMe3 | DMAP | ||||
| 1 | O,NTs | Ph, H (2c) | 4i | 83 | |
| 2 | O,NTs | Bn, H (2d) | 4j | 61 | 77 |
| 3 | O,NTs | t-Bu, H (2e) | 4k | 69 | 51 |
| 4 | O,S | Me,H | 4l | 74 | 76 |
| 5 | O,S | Ph,H | 4m | 86 | |
| 6 | O,S | Bn,H | 4n | 65 | 89 |
| 7 | O,S | t-Bu, H | 4o | 58 | 48 |
| 8 | O,O | Me,H | 4p | 70 | 68 |
| 9 | O,O | Ph,H | 4q | 77 | |
| 10 | O,O | Bn,H | 4r | 89 | 74 |
| 11 | O,O | t-Bu, H | 4s | 82 | 68 |
| 12 | O,O | H, Me (2f) | 4t | 89 | |
| 13 | O,O | H, Bn (2g) | 4u | 86 | |
| 14 | O,O | H, CH2CO2Et(2h) | 4v | 80 | |
| 15c | O,NTs | H,Me | 4w | 81d | |
| 16c | O,NTs | H,Bn | 4x | 73d | |
| 17c | O,NTs | H, CH2CO2Et | 4y | 84d | |
Reactions were performed using 0.4 mmol of 1 and 1.35 equiv of 2.
Isolated yield.
NaOAc (50 mol %) was added.
Diastereoisomeric ratio determined using 1H NMR spectroscopy. Diastereomeric ratios 1:1, 2:1, and 1.2:1 for 4w, 4x, and 4y, respectively.
