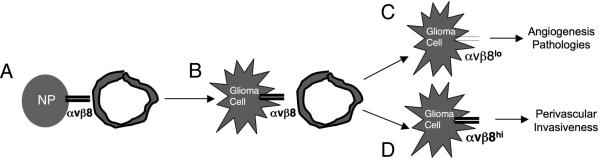
Figure 6. A model for β8 integrin-mediated regulation of GBM angiogenesis versus tumor cell invasiveness.
(A); In the normal brain astrocytes and neural progenitor cells (NP) express αvβ8 integrin and regulate cerebral blood vessel (BV) development and physiology via activation of TGFβ signaling pathways. (B); Genetic mutations in NPs induce their transformation and initiate development of astrocytomas that maintain αvβ8 integrin expression. (C, D); As astrocytomas progress to GBM, sub-populations of tumor cells express different levels of αvβ8 integrin protein. Cells with low αvβ8 integrin protein (αvβ8lo) activate diminished levels of TGFβs and contribute to angiogenesis and vascular permeability pathologies (C). In contrast, cells that express elevated levels of αvβ8 integrin protein (αvβ8hi) activate more TGFβs and drive invasive GBM growth properties (D). Differential levels of integrin-mediated TGFβ activation and signaling likely cooperate with the Hif1α/VEGF-A pathway to regulate angiogenesis versus tumor cell invasiveness.