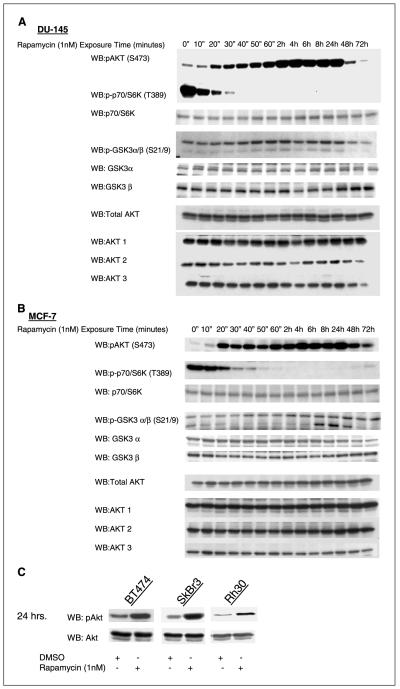
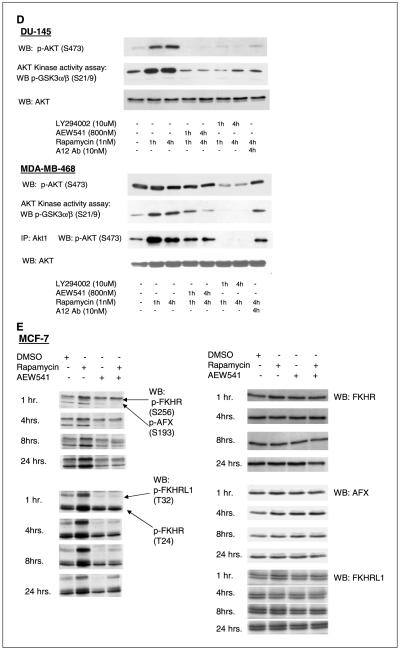
Figure 1.
mTOR inhibition activates Akt in tumor cells. A, 1 nmol/L rapamycin treatment induced S473 Akt and S21/9 GSK3α/β phosphorylation in vitro in a DU-145 prostate cancer cell line. Akt1, Akt2, and Akt3, total Akt, and total GSK3α/β did not change. Phosphorylation of p70/S6K decreased with rapamycin treatment whereas total p70/S6K levels did not change. B, 1 nmol/L rapamycin treatment induced S473 Akt and S21/9 GSK3α/β phosphorylation in vitro in a MCF-7 cancer cell line. Akt1, Akt2, and Akt3, total Akt, and total GSK3α/β did not change. Phosphorylation of p70/S6K decreased with rapamycin treatment whereas total p70/S6K levels did not change. C, 1 nmol/L rapamycin treatment for 24 hours induced S473 Akt in the breast cancer cell lines BT474, SkBr3, and the rhabdomyosarcoma cell line Rh30. Total Akt levels did not change.
D, mTOR inhibition with 1 nmol/L rapamycin induced Akt Kinase activity in an IGF-IR-dependent manner. An Akt kinase assay in DU-145 cells confirmed that the increased pAkt (S473) resulted in increased Akt activity, which was abrogated by the inhibition of PI3K with the small-molecule LY294002 (10 μmol/L) and by inhibition of IGF-IR with either the small-molecule NVP-AEW541 (800 nmol/L) or the monoclonal antibody A12 (10 nmol/L). A Western blot of pAkt (S473) at the same time points in DU-145 showed the increased Akt phosphorylation which was abrogated by IGF-IR or PI3K inhibition. Although there is no detectable increase in pan-phospho-Akt as measured by Western blot, an Akt kinase assay in MDA-MB-468 cells reveals an induction of Akt activity and p-Akt1 (S473) that is abrogated by LY294002 as well as the IGF-IR inhibitors. E, treatment of MCF-7 cells with rapamycin (1 nmol/L) induced phosphorylation of the FoxO transcription factors, FKHR (S256 and T24), AFX (S193), and FKHRL1 (T32). The rapamycin-induced FoxO phosphorylation was abrogated by IGF-IR inhibition with 800 nmol/L NVP-AEW541. Total FKHR, AFX, and FKHRL1 levels did not change with rapamycin or NVP-AEW-541 treatment.