Abstract
Nocardia is a rare pathogen of peritoneal dialysis-related peritonitis despite its universal presence in soil, organic matter, and water. Human infections are seen mostly in immunocompromized hosts. The usual primary site of lesions is lung. Nocardia can enter the peritoneal cavity through the Tenckhoff catheter. We describe the first case of nocardia peritonitis that had associated nocardia pneumonia. The Tenckhoff catheter was removed due to nonresolution of peritonitis. The organism was resistant to sulfamethoxazole–trimethoprim. The patient developed hypotension, disseminated intravascular coagulation, and respiratory failure and died.
Keywords: Nocardia asteroides, peritoneal dialysis, peritonitis
Introduction
Peritonitis remains a leading cause of hospitalization, technique failure, and mortality in continuous ambulatory peritoneal dialysis (CAPD) patients, despite the advancement made in the technology of solution delivery systems, exit-site care, and training protocols of PD. Nocardia is a rare pathogen of PD-related peritonitis despite its universal presence in soil, organic matter, and water. Human infections are seen mostly in immunocompromised hosts. The primary site of lesions is usually lung from where it spreads to other organ systems.[1] Pneumonia and disseminated disease are common presentations. Reports of nocardia causing PD-related peritonitis are rare.[2] The predisposing factors, treatment protocol, and whether to treat with or without catheter in situ are unanswered questions in nocardia peritonitis. We report a CAPD patient who developed peritonitis due to Nocardia asteroides and reviewed the literature related to this organism causing peritonitis and outcome of this rare infection.
Case Report
A 57-year-old male, diabetic end-stage renal disease patient was started on CAPD in January 2008. A double-cuff, straight Tenckhoff catheter was inserted under local anesthesia and PD was started after a break-in period of 2 weeks. His dialysis prescription was three 2-l exchanges daily (two exchanges of 6-h dwell with 1.5% dextrose and night dwell with 2.5% dextrose (Dianeal, Baxter India, Gurgaon, India). He developed two episodes of peritonitis, an episode of Staphylococcus epidermidis peritonitis after 6 months and second episode of culture-negative peritonitis 10 months after the start of PD. Both episodes were treated with intraperitoneal cefazolin and tobramycin, and they resolved after treatment.
In July 2009, he presented with fever, cough, and sputum for 1 week. There was no history of cloudy effluent and any other history suggestive of peritonitis. On examination, the blood pressure was 126/74 mmHg, respiratory rate 20/min, pulse 80/min, and temperature 38.1°C. Chest examination revealed right upper and mid-zone rales. Rest of the physical examination was within normal limits. His laboratory parameters revealed a total white cell count of 12,500/cmm, hemoglobin 11.3 g/dl, platelets 240,000/cmm, serum albumin 2.6 g/dl, and dialysate cell count 20/mm3 (90% lymphocytes). Chest X-ray revealed pneumonitis and cavity in the right upper and mid-zone of the lung [Figure 1]. He was started on intravenous amoxicillin-sulbactum and cefoperazone and fluconazole as antifungal prophylaxis as per our institute protocol. His sputum grew nocardia after 96 h of incubation. The organism was identified as N. asteroides based on hydrolysis tests for casein, tyrosine, xanthine, and hypoxanthine. Four days after initiation of antibiotic therapy, the patient developed abdominal pain, cloudy effluent, and rebound abdominal tenderness. The effluent fluid total leukocyte count was 320 cells/cmm with 90% neutrophils. Intraperitoneal vancomycin and ceftazidime were administered. The Gram stain did not reveal any organisms and the first culture of the PD fluid turned to be sterile; however, the effluent remained cloudy after 5 days of therapy. The second Gram stain revealed Gram-positive extensively branching bacilli that fragmented into rod-shaped to coccoid form and were found to be acid-fast by the modified Kinyoun method [Figure 2a]. On culture, chalky white colonies were seen on blood agar [Figure 2b]. Based on hydrolysis tests for casein, tyrosine, xanthine, and hypoxanthine, the organism was identified as N. asteroides. Repeat dialysate culture also grew N. asteroides.
Figure 1.
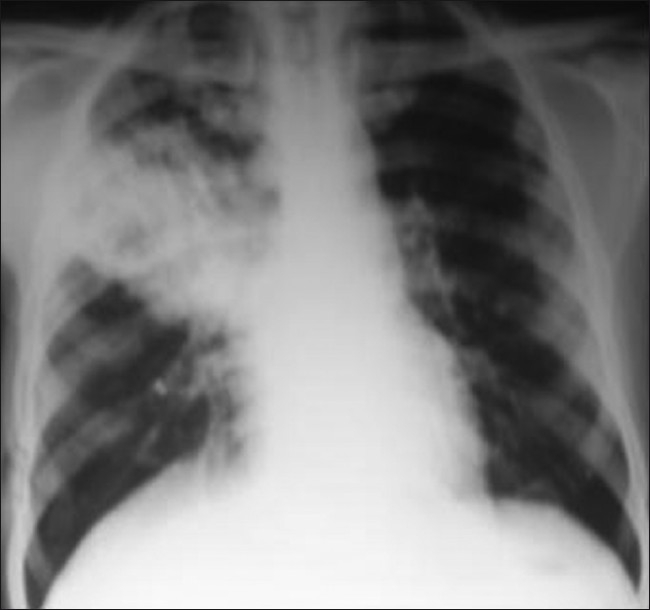
Chest X-ray showing pneumonia and a cavity in the right mid- and upper zone of chest
Figure 2.
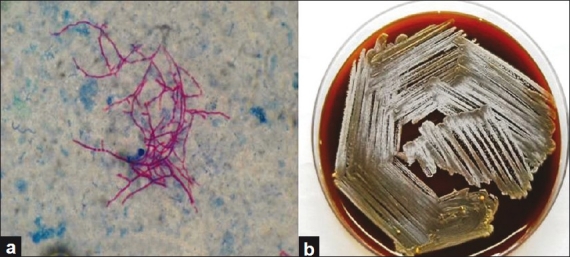
(a) Peritoneal dialysis effluent showing acid-fast branching of bacilli that fragment to the coccobacillary form (magnification, ×100). (b) Chalky white colonies of Nocardia asteroides on the blood agar plate
The patient was started on intraperitoneal trimethoprim–sulfamethoxazole combination (TMP–SMX). After 4 days of treatment with TMP–SMX, the effluent remained cloudy with the effluent fluid cell count 800 cells/cmm (80% neutrophils) and hence the Tenckhoff catheter was removed. The intra-abdominal segment of the catheter was sent for the culture which also grew N. asteroides. The isolate was resistant to TMP–SMX and hence intravenous ceftriaxone and ciprofloxacin were started as per the sensitivity report. Dialysate microscopy and cultures for fungus and tuberculosis were sterile. Blood and urine cultures were sterile. The patient was started on maintenance hemodialysis. Subsequently, only during this hospital stay after 2 weeks, the patient developed hypotension, disseminated intravascular coagulation, and respiratory failure, and died.
Discussion
This patient died of nocardia peritonitis. The identification was delayed due to rarity of the organism. Fungal, mycobacterium infections, and intra-abdominal abscess are usually looked for if the dialysate count and peritonitis symptoms fail to improve within 3–4 days but nocardia is not a usual organism causing peritonitis. This is the first case of nocardia peritonitis in PD practice of more than two decades at our institute. It is possible that some of the episodes remained undiagnosed among culture-negative peritonitis which constitutes about 20% of all episodes of peritonitis. Nocardia is a slow-growing organism and may take up to 4 weeks to grow from the clinical material, while 4–10 days is normal.[3] Unless specifically sought by prolonged incubation, it may be missed. Owing to the tendency to form clumps in a liquid medium, nocardia is hard to disperse uniformly. It may be responsible for the initial failure to isolate it until the second centrifuged dialysate sample was examined. It is also a strict aerobe and grows poorly in the reducing atmosphere provided by the thioglycollate broth, which is normally an excellent medium for recovering the organisms encountered in CAPD peritonitis.[3]
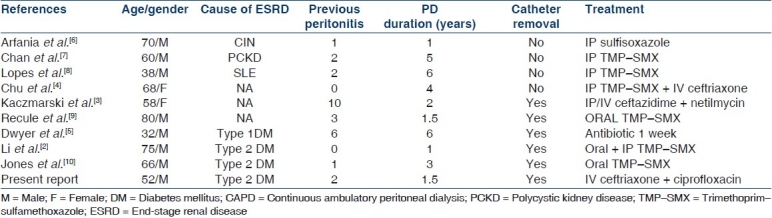
Nocardia is a saprophytic, soil microflora associated with water and dust. It is a Gram-positive bacillary branching bacterium whose hyphae often fragment to coccobacillary forms. A recent application of modern taxonomic procedures, including more extensive phenotypic evaluation, molecular characterization, and numerical taxonomic methods, has expanded our knowledge of its phylogenetic and taxonomic status. Most nocardia infections in humans are caused by N. asteroides, N. nova, N. farcinica, and N. brasiliensis.[1] Human infection occurs primarily in immunocompromised individuals as an opportunistic infection. It usually originates in the lungs and tends to spread to other organ systems, most commonly the brain and skin and rarely to the kidneys, joints, heart, eyes, and bones. A smaller number of infections are caused by traumatic percutaneous introduction. Before our report, there have been nine case reports of nocardia peritonitis in the literature [Table 1] but, none had previously mentioned pulmonary or systemic nocardia infection. Li et al.[2] thought that nocardia is introduced into the peritoneal cavity via the PD catheter rather than the blood stream. Our case was different because this patient had pneumonia at presentation and the growth of nocardia on the sputum culture confirmed nocardia infection. This is probably the first case of nocardia CAPD peritonitis secondary to dissemination from pulmonary focus. Nocardia peritonitis has been reported in both diabetic and nondiabetic patients and diabetes does not appear to be the risk factor. The cases have been reported in different time frames of 1–6 years from the start of PD. Our patient developed nocardia peritonitis after 2 years of PD. All patients but two had previous episodes of peritonitis ranging from 1 to 10 episodes, and the interval of nocardia peritonitis from the last episode of peritonitis was 2 weeks to 5 months. Our patient also had two episodes of peritonitis before developing nocardia peritonitis and had a gap of 8 months after the last episode of peritonitis. Reviews of case reports suggest that the previous episodes of peritonitis may be one of the risk factors for nocardia peritonitis.
Table 1.
Summary of review of case reports of nocardia peritonitis in CAPD patients
TMP–SMX is the drug of choice, used alone or in combination with other antimicrobials, for systemic nocardiosis. Severe disease requires combination therapy of sulfonamides, amikacin and imipenam. Although sulfonamide resistance is rare but it is not uncommon in India due to its widespread use and free over-the-counter availability. Our patient was also resistant to TMP–SMX. This was the probable reason for a poor response to the intraperitoneal administration of TMP–SMX in our patient. Cephalosporins, minocycline, fluoroquinolones, and macrolides have all been successfully used to treat nocardiosis. Therefore, the choice of antibiotic should be based on the local resistance and sensitivity pattern of the organism in that institute. The duration of therapy is uncertain, but needs to be protracted because of the host's compromised immunity and the occurrence of considerable numbers of relapses after shorter courses of therapy. From the limited published experience, 3- to 6-week IP administration of sulfonamides may preserve the CAPD catheter in nondiabetic patients. Recurrent episodes of peritonitis might develop with a shorter duration of therapy. Chu et al.[4] reported that the dialysate count of a CAPD patient with nocardia peritonitis decreased on IP TMP–SMX but the patient died of myocardial infarction before the antibiotic treatment was completed. One patient developed intra-abdominal abscess and needed surgical drainage, whose catheter was removed after nocardia peritonitis without preceding IP sulfa-containing antibiotic treatment.[5] Our patient died despite the catheter removal because of concomitant nocardia peritonitis and pneumonia. The decision of catheter removal is always crucial in the management of peritonitis. Successful treatment of nocardia peritonitis has been reported with and without PD catheter removal. A total of 6 of the 10 patients reported so far had their catheters removed. Li et al.[2] have reported a patient successfully treated with intraperitoneal TMP–SMX; however, catheter removal was done 6 weeks later due to relapsing peritonitis. Kaczmarski et al.[3] also reported late identification of the organism, an initial good response after 7 days of therapy but relapse after 12 days of therapy. From the limited experience of the case report and review it appears that if IP administration of TMP–SMX fails to relieve peritonitis symptoms and normalize the dialysate leukocyte count completely, the removal of the PD catheter and prolonged antibiotic treatment remains the only reliable therapeutic modality.
We conclude that nocardia peritonitis should be suspected in patients with concomitant lung infection and culture-negative peritonitis Tenckhoff catheter removal should be considered in patients not responding to the intraperitoneal administration of antibiotics for 4–5 days. The drug of choice should be determined based on the local sensitivity pattern.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Saubolle MA, Sussland D. Nocardiosis: review of clinical and laboratory experience. J Clin Microbiol. 2003;41:4497–501. doi: 10.1128/JCM.41.10.4497-4501.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li SY, Yu KW, Yang WC, Chen TW, Lin CC. Nocardia peritonitis - a case report and literature review. Perit Dial Int. 2008;28:544–7. [PubMed] [Google Scholar]
- 3.Kaczmarski EB, Wilkie M, Thornhill C, Oppenheim BA, Ackrill P. Problems encountered in diagnosis of Nocardia asteroides peritonitis complicating CAPD. Perit Dial Int. 1990;10:106. [PubMed] [Google Scholar]
- 4.Chu KH, Fung KS, Tsang WK, Chan HW, Tong KL. Nocardia peritonitis: satisfactory response to intraperitoneal trimethoprim-sulfamethoxazole. Perit Dial Int. 2003;23:197–8. [PubMed] [Google Scholar]
- 5.Dwyer KM, Daffy JR, Murphy BF. Nocardia peritonitis and abdominal abscess complicating continuous ambulatory peritoneal dialysis. Nephrology. 2001;6:263–5. [Google Scholar]
- 6.Arfania D, Everett ED, Nolph KD, Rubin J. Uncommon causes of peritonitis in patients undergoing peritoneal dialysis. Arch Intern Med. 1981;141:61–4. doi: 10.1001/archinte.141.1.61. [DOI] [PubMed] [Google Scholar]
- 7.Chan DT, Cheng IK, Chan PC, Mok KY. Nocardia peritonitis complicating continuous ambulatory peritoneal dialysis. Perit Dial Int. 1990;10:99. [PubMed] [Google Scholar]
- 8.Lopes JO, Alves SH, Benevenga JP, Salla A, Tatsch I. Nocardia asteroides peritonitis during continuous ambulatory peritoneal dialysis. Rev Inst Med Trop Sao Paulo. 1993;35:377–9. doi: 10.1590/s0036-46651993000400013. [DOI] [PubMed] [Google Scholar]
- 9.Recule C, Milongo R, Boiron P, Croize J. Nocardia peritonitis complicating CAPD. Perit Dial Int. 1994;14:297–8. [PubMed] [Google Scholar]
- 10.Jones KJ, Ratanjee SK, Taylor SL, Marshall MR. Nocardia asteroides peritoneal dialysis-related peritonitis: a case of successful treatment and return to peritoneal dialysis. Nephrol Dial Transplant. 2008;23:2693–4. doi: 10.1093/ndt/gfn252. [DOI] [PubMed] [Google Scholar]


