Abstract
Background
Little is known about the prevalence of self-reported photosensitivity and its effects on quality of life in a U.S. cutaneous lupus population
Objective
We sought to determine the prevalence of self-reported photosensitivity among a cutaneous lupus population and to examine its impact on quality of life
Methods
169 subjects with lupus were interviewed about photosensitivity symptoms and completed the modified Skindex-29+3, a quality of life survey. A complete skin exam was conducted and the Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI) was completed.
Results
68% of subjects reported some symptoms of photosensitivity (PS). The PS group (subjects who reported a history of and current photosensitivity) scored worse on photosensitivity-related items of the modified Skindex-29+3 and had higher cutaneous disease activity as determined by the CLASI. Photosensitive patients had worse symptoms and emotions and experienced significant functional impairments compared to patients with cutaneous lupus but without photosensitivity.
Limitations
This study was done at a single-referral center
Conclusions
Self-reported photosensitivity is very common among cutaneous lupus patients and is associated with significant impairments related to symptoms, emotions, and daily functioning.
Keywords: photosensitivity, quality of life, cutaneous lupus erythematosus, CLASI, UVR, mental health, functional status
INTRODUCTION
Ultraviolet radiation (UVR) may play a role in the development and exacerbation of lupus erythematosus (LE). Because cutaneous manifestations of LE often arise in sun-exposed areas and exposure to environmental UVR can elicit skin lesions, patients with LE are often labeled ‘photosensitive’1–4. The term ‘photosensitivity’, however, is ill-defined and is often used to describe a variety of reactions to UVR5.
The American College of Rheumatology (ACR) defines photosensitivity, one of the criteria for diagnosis of systemic lupus erythematosus (SLE), as “an unusual reaction to sunlight by a patient’s history or by physician observation”6. This definition is very broad and encompasses both self-reported symptoms and clinically apparent reactions to UVR. Provocative phototesting has been used to test photosensitivity in a more objective manner. A positive reaction to phototesting that is clinically, morphologically, and histologically consistent with lupus and occurs in the typical delayed time course attempts to define photosensitivity among a LE population3,7. This measure of photosensitivity, however, is influenced by a variety of factors including but not limited to type and amount of UVR, site and size of skin exposure, and patient skin type. Moreover, results of provocative phototesting are often incongruent with a patient’s history of photosensitivity5,8,9.
Not surprisingly, the prevalence of photosensitivity in LE patients varies widely depending on the definition employed, the LE subtype, race, and geographical location10–12. According to the ACR definition of photosensitivity, 57–73% of SLE, 50–90% of subacute cutaneous lupus erythematosus (SCLE), approximately 50% of discoid lupus erythematosus (DLE), and nearly all tumid lupus erythematosus (LET) patients are photosensitive8,13–21. Similarly, broad prevalence figures for photosensitivity assessed by provocative phototesting have been reported, which range from 10–74% for SLE, 50–100% for SCLE, 16–64% for DLE, and 76–81% for LET2,3,22–27.
Irrespective of patient history or phototesting assessment, LE patients are advised to avoid sunlight to prevent lupus flares. Wearing long clothes and a hat year-round, avoiding sun between 10 am and 2 pm, and reapplying sunscreen several times throughout the day are not trivial tasks. Compliance with sun avoidance limits patients’ ability to take part in day-to-day activities, hobbies, and social gatherings, which can significantly impact quality of life. In a study of SLE patients in the U.S., over one-third of patients reported that photosensitivity (by patient assessment using a visual analogue scale) had a significant impact on their quality of life14.
In this cross-sectional analysis of an ongoing database study, we sought to determine the prevalence of self-reported photosensitivity in a U.S. population of primarily cutaneous lupus erythematosus (CLE) patients and to examine the impact of photosensitivity on quality of life. Secondary objectives were to begin to validate clinical interview questions used to ascertain self-reported photosensitivity and the modified Skindex-29+3 photosensitivity items and PS subscale, and to examine the relationship between photosensitivity and cutaneous lupus disease activity using the CLASI.
METHODS
Subject selection
Patients with LE presenting to the outpatient medical dermatology clinic at the University of Pennsylvania were consecutively enrolled in our ongoing database study on the prevalence and severity of lupus erythematosus. All patients over 18 years of age with clinical, histological, and/or serological evidence of cutaneous lupus and/or systemic lupus erythematosus with skin manifestations were invited to participate. Subjects were categorized according to the modified Gilliam classification28 into the various subtypes of CLE: acute cutaneous lupus erythematosus (ACLE), SCLE (annular or papulosquamous), and chronic cutaneous lupus erythematosus (CCLE) (classic DLE [generalized or localized], hypertrophic DLE, LET, chilblains, or lupus panniculitis). Subjects with SLE who met the American Rheumatism Association/ACR criteria6 were included if they also had a form of CLE or had lupus nonspecific skin manifestations (including but not limited to livedo reticularis, Raynaud’s phenomenon, ulceration). The protocol for the study was approved by the institutional review board of the University of Pennsylvania School Of Medicine and was in accordance with the Declaration of Helsinki in its current form. All subjects were consented by means of institutional review board–approved informed consent and Health Insurance Portability and Accountability Act forms.
Study procedures
Study visits were completed at the time of the subject’s regularly scheduled clinic visit. Every effort was made to conduct a study visit at the time of enrollment. On occasions when this was not feasible, the first study visit was completed at the next scheduled clinic visit. Thereafter, study visits were conducted as often as the subject was willing or if in the interim since the last study visit any of the following criteria were met: 1) the subject had a change in symptoms (disease significantly worsen or improved) 2) the subject had a change in medication (started or stopped a medication) or 3) it had been greater than 1 year since the last study visit.
Information was obtained by patient history, physical examination, medical record review, and subject questionnaires. Immediately prior to the clinic visit, subjects were given quality of life questionnaires, including the modified Skindex-29+3 to complete in the waiting area. During the study visit, sociodemographic information and medical history was collected. The subject was interviewed about smoking and sun exposure, SLE symptoms, comorbid autoimmune conditions, medication effectiveness, and side effects. A complete skin examination was performed and the cutaneous lupus erythematosus disease area and severity index (CLASI) outcome measure was completed. Whenever available, recent laboratory values, including lupus serologies and/or biopsy results, were reviewed and documented.
Photosensitivity items and visit selection
At each visit, subjects were asked the following two questions to ascertain self-reported photosensitivity:
Do you have a history of photosensitivity?
Since the last visit, have you been experiencing sensitivity to sunlight?
Any adverse reaction to sunlight reported by the subject was recorded as a ‘yes.’ Subject reports of sensitivity to sunlight included but were not limited to: sun brings out my lupus lesions, sun causes me to get a rash, I feel sick in the sun, and my skin tingles in the sun. Based on answers to these two photosensitivity questions, subjects were classified into one of three photosensitivity groups: Photosensitive (PS) group, PS Suggestive group, and NOT PS group. To avoid overrepresentation by one subject, only one visit per subject was selected for analysis. (Table 1). In addition to the above photosensitivity questions, at each visit subjects were asked about the frequency with which they used sun protection and development of lupus lesions in sun-exposed areas:
Table 1.
Photosensitivity classification and visit selection
| History of Photosensitivity | Current Photosensitivity symptoms | Visit Selected for analysis | Photosensitivity Grouping | |
|---|---|---|---|---|
| At 1 or more visits, subject answered: | Yes | Yes | First visit in which subject answered ‘yes’/’yes’ | PS group |
| If subject never answered ‘yes’/’yes’, but answered ‘yes’ to either question at 1 or more visits: | Yes | No | First visit in which subject answered ‘yes’/’no’ or ‘no’/’yes’ | PS Suggestive group |
| No | Yes | |||
| If subject never answered ‘yes’ to either question at any visit: | No | No | First visit in which subject answered ‘no’/’no’ | NOT PS group |
Do you protect your skin from the sun with sun protective clothing and/or sunscreen? [answer options: daily, usually, sometimes, rarely, never]
Do you have lesions in sun-exposed areas? [answer options: yes, no]
Modified Skindex-29+3
The Skindex-29 is a validated measure of skin-specific quality of life29–32. The impact of skin on functioning, symptoms, and emotions is assessed by self-report. The level of agreement with items corresponding to the 3 subscales is assessed on a 5-point Likert scale (never, rarely, sometimes, often, all the time). Individual items are scored from 0 – 100 in 25 point increments with 100 representing maximal disability. The subscales are determined by taking the mean of the items that represent that subscale. Three lupus specific items were added to the Skindex-29 to create the modified Skindex-29+333. Two items, “I worry about going outside because the sun might flare my disease” and “My skin disease prevents me from doing outdoor activities” relate to photosensitivity. The average of these two items was used to generate a photosensitivity subscale (PS scale).
Cutaneous lupus erythematosus disease area and severity index (CLASI)
The CLASI is a validated tool to assess disease severity in cutaneous lupus erythematosus34–37. It quantifies disease activity (erythema, scale) and damage (dyspigmentation, scar) over 13 distinct areas of the body. Activity and damage scores range from 0–70 and 0–56 respectively, with higher scores representing more severe disease. Disease activity is classified into mild (0–9) and moderate-to-severe (>/= 10) by CLASI activity score.
Data collection
Data were collected in accordance with good clinical practice guidelines to ensure accuracy and integrity. Completeness of data and use of explicit definitions for variables were assessed and a constant effort at quality control was maintained. Data were then organized and entered into a collaborative web-based database. Data security and confidentiality were managed carefully to ensure regulatory adherence.
Statistical Analysis
Descriptive statistics including the frequency, means, and standard deviations of outcome variables were generated. Contingency tables with Pearson’s chi-square analyses were used to compare frequencies between groups. Student’s t-tests were used to determine mean differences in CLASI activity scores between groups. Mean differences in the photosensitivity items and PS scale of the modified Skindex-29+3 were subjected to analysis of variance (ANOVA). Mean differences in the 3 modified Skindex-29+3 scales (symptoms, emotions, function) were compared by two-factor (photosensitivity, CLASI activity) ANOVA. The relationship between photosensitivity, CLASI activity, and quality of life measures on the Skindex were further analyzed by stratifying the PS group into Hi PS/Low PS and Mild Activity/Moderate-Severe Activity. The Hi PS group was comprised of subjects scoring greater than the mean PS scale score and the Low PS group scored less than the mean. Mild Activity group was classified as ≤ 9 CLASI activity and the Moderate-Severe Activity group was comprised of subjects scoring > 9 on the CLASI38. Mean Skindex scores within the four PS group strata were subjected to ANOVA. Two-tailed tests of significance level with type I (α) error rate of 0.05 were utilized.
RESULTS
A total of 169 subjects were enrolled in the study. 80% were women and 20% were men. Nearly half of the sample was diagnosed with DLE (46%), 25% with SCLE, 9% with LET, 7% with ACLE, 7% with SLE and nonspecific skin manifestations, 4% had >1 CLE subtype, and 2% had other forms of chronic CLE ([other CCLE], including panniculitis or chilblains). The >1 CLE subtype category was comprised of 4 subjects with SCLE and DLE and one of each with SCLE and ACLE, SCLE and LET, and DLE and panniculitis. Two-thirds of subjects were Caucasian, 28% were African American, 5% were Asian, and 1 subject was Hispanic (Table 2).
Table 2.
Subject characteristics
| N | Percent | ||
|---|---|---|---|
| Gender | |||
| Male | 33 | 20 | |
| Female | 136 | 80 | |
| Diagnosis | |||
| DLE | 77 | 46 | |
| SCLE | 42 | 25 | |
| LET | 16 | 9 | |
| ACLE | 11 | 7 | |
| SLE nonspecific | 12 | 7 | |
| >1 CLE subtype | 7 | 4 | |
| CCLE other | 4 | 2 | |
| Race | |||
| Caucasian | 111 | 66 | |
| African-American | 48 | 28 | |
| Asian | 8 | 5 | |
| Hispanic-Latino | 2 | 1 | |
| Total | 169 | ||
DLE – discoid lupus erythematosus; SCLE – subacute cutaneous lupus erythematosus; LET – tumid lupus erythematosus; SLE nonspecific – subjects with nonspecific skin manifestations (e.g. livedo reticularis) that met criteria for systemic lupus erythematosus; >1 CLE subtype – subjects having more than one type of CLE [4 with SCLE+DLE; 1 with SCLE+ACLE; 1 with ACLE+LET; 1 with DLE+ panniculitis]
Prevalence of photosensitivity
Of the 169 subjects, 91 had both a history of and ongoing photosensitivity, 11 had a history of photosensitivity, 13 had new onset photosensitivity, 40 did not experience photosensitivity, and in 14 subjects we were unable to determine photosensitivity status because of incomplete information. Overall, 68% of the sample reported symptoms of photosensitivity while only 24% of subjects denied photosensitivity. Approximately 82% of ACLE, 76% of SCLE, 71% of >1 CLE subtype, 63% of LET, 58% of SLE with nonspecific skin manifestations, 46% of DLE, and 0% of other CCLE patients (panniculitis or chilblains) reported both a history of and current photosensitivity (and were classified into PS group) (Figure 1).
Figure 1. Prevalence of photosensitivity within each LE type.
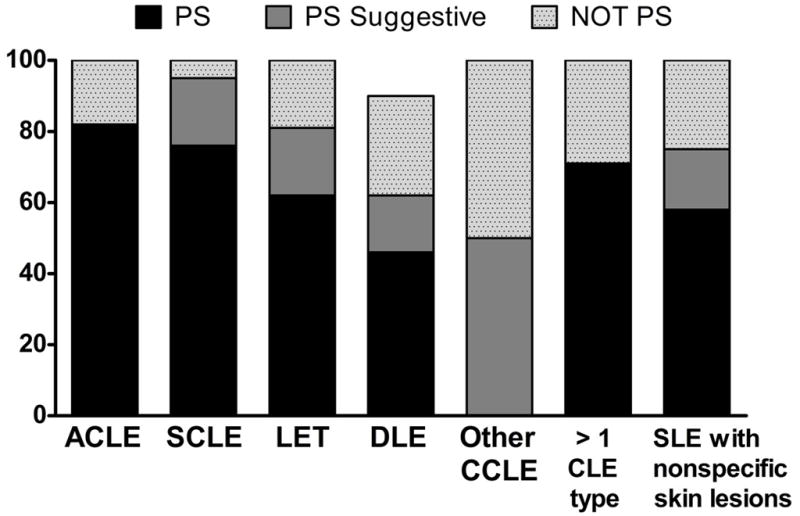
Over 75% of ACLE, SCLE, and >1 CLE subtype (comprised mostly of SCLE + another type of CLE) were photosensitive while subjects with DLE were least photosensitive with a prevalence of 45%.
PS group characteristics
Of the 91 subjects comprising the PS group, 35% had DLE, 31% had SCLE, 11% had LET, 10% had ACLE, 8% had SLE with nonspecific skin manifestations, and 5% had >1 CLE subtype. There was a significant association between two lupus subtypes (DLE, SCLE) and photosensitivity grouping (Pearson’s X2 = 17.92; p = 0.006). SCLE patients were more likely to be categorized into the PS group while DLE patients were more likely to be classified into the NOT PS group. There was no significant relationship between any of the other LE diagnoses (ACLE, LET, SLE with nonspecific skin manifestations, >1 CLE subtype, and other CCLE) and photosensitivity grouping.
Sun exposure-related characteristics in PS group vs. NOT PS group
The PS group developed lesions in sun-exposed areas significantly more frequently than the NOT PS group (71% vs 31%, X2 = 5.88, p=0.015). The PS group engaged in sun protective behaviors significantly more frequently than the NOT PS group (X2 = 16.63, p = 0.002).
Photosensitivity items of the Modified Skindex-29+3
ANOVA showed that the effect of photosensitivity was significant for Item 31 (F=19.51, p=0.000), Item 33 (F=16.18, p=0.000), and the PS scale (F=21.58, p=0.000). The PS group scored significantly worse on Item 31: I worry about going outside because the sun might flare my disease (M=74.2, SD=28.9 vs. M= 40, SD=29.3 in NOT PS group), and on the PS scale (average of the items 31 and 33) (M=55.7, SD=27.1. vs. M= 35.9, SD=26.4 in NOT PS group) compared with the NOT PS group. For Item 33: My skin disease prevents me from doing outdoor activities, the PS group scored significantly worse (M=65.9, SD=33.2) than the PS Suggestive (M=46.8, SD=34.0) and even more so compared with the NOT PS group (M= 31.8, SD=28.9) (Figure 2).
Figure 2. Mean scores for photosensitivity items of Modified Skindex-29+3.
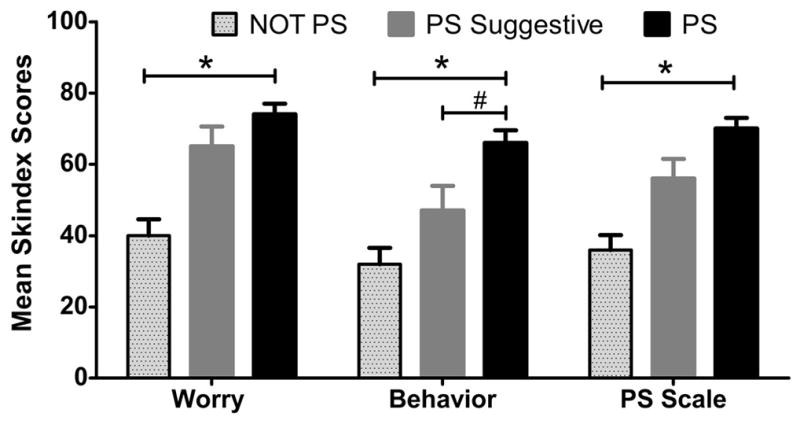
The mean scores (mean + SEM) for photosensitivity items and photosensitivity subscale (PS Scale) by photosensitivity grouping. * indicates significant differences between the PS group and the NOT PS group; p< 0.05 with Bonferroni correction after ANOVA. # indicates significant differences between the PS group and the PS Suggestive group; p< 0.05 with Bonferroni correction after ANOVA.
CLASI activity and photosensitivity
ANOVA showed that the effect of photosensitivity was significant for CLASI activity score (F=8.30, p=0.005). Subjects in the PS group had significantly increased lupus specific cutaneous disease activity as measured by the CLASI activity score compared with subjects in the NOT PS group (M=9.2, SD=9.4 vs. M=4.6, SD=5.6). A linear regression analysis with CLASI activity as the response variable and photosensitivity (yes/no) as the independent variable was statistically significant (F = 8.30, p =0.005). CLASI activity was moderately correlated (r=0.36) with the presence/absence of photosensitivity (Table 2).
Effect of photosensitivity and CLASI activity on Modified Skindex 29+3 subscales
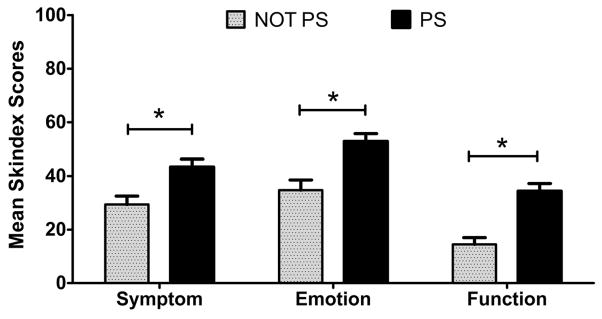
Two-way ANOVA yielded a main effect for photosensitivity (yes vs. no), on all three subscales of the modified Skindex-29+3, symptom (p=0.007), emotion (p=0.003), and function (p=0.000) such that the PS group had significantly worse scores on average compared with the NOT PS group (Figure 3). A main effect for CLASI activity [mild (0–9) vs. moderate and severe (>9)] was also significant for the symptom (p=0.007) and emotion subscales (p=0.003) but not for the function subscale (p=0.10) of the modified Skindex-29+3 such that the subjects with more severe (higher) CLASI activity scores had significantly worse scores on average compared with subjects with mild (lower) CLASI activity scores.
Figure 3. Mean Modified Skindex-29+3 subscale scores between the PS and NOT PS group.
The mean scores (mean + SEM) for the modified Skindex-29+3 subscales (emotion, symptoms, functioning) subscales. * the PS group scored worse on all three subscales of the modified Skindex-29+3 with p< 0.05.
Within PS Group strata: Hi/Low PS and Mild/Moderate-Severe CLASI activity
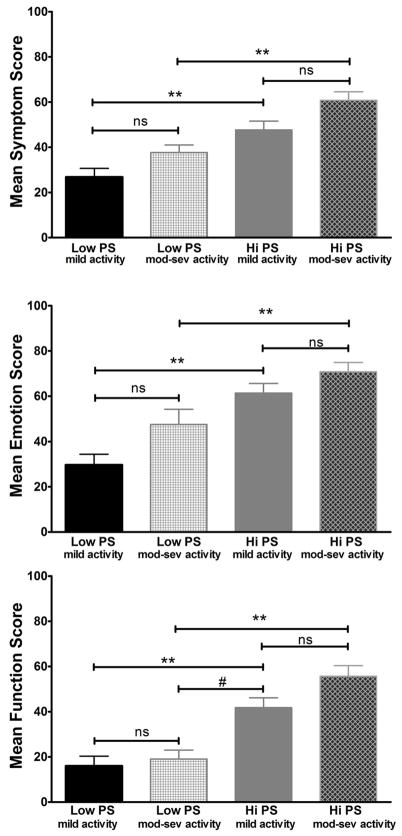
ANOVA showed that the effect of PS group strata was significant for symptom, emotion, and function (F=11.90, p=0.000; F=13.76, p=0.000; F=14.97, p=0.000). Post hoc analyses using Bonferroni criterion for significance showed that the Hi PS group scored significantly worse on the symptom scale compared to the Low PS group within each disease activity strata [(Mild Activity Group: Low PS M=26.8, SD=19.2 vs. Hi PS M=47.7, SD=22.9, p=0.001)(Mod-Severe Activity Group: Low PS M=37.7, SD=12.5 vs. Hi PS M=60.8, SD=16.7, p=0.006)]. The same was true for emotions with the Hi PS group scoring significantly worse than the Low PS group within each disease activity strata [(Mild Activity Group: Low PS M=29.7, SD=23.0 vs. Hi PS M=61.4, SD=24.5, p=0.000)(Mod-Severe Activity Group: Low PS M=47.5, SD=25.0 vs. Hi PS M=70.8, SD=18.1, p=0.031)]. In terms of functioning, the Hi PS group scored significantly worse than the Low PS group within and across activity strata such that the Hi PS, Mild Activity group scored worse than the Mod-Severe Activity, Low PS group (Hi PS, Mild Activity M=41.8, SD=25.5 vs. Low PS, Mod-Sev Activity M=19.0, SD=14.8, p=0.010) (Figure 4).
Figure 4. Mean Modified Skindex-29+3 subscale scores across Hi/Low PS groups and Mild Activity/Moderate-Severe Activity strata.
The mean scores (mean + SEM) for the modified Skindex-29+3 subscales (emotion, symptoms, functioning) subscales. ** the HI PS group scored worse than the Low PS group within each disease activity strata on all three subscales of the modified Skindex-29+3 with p< 0.01. # the Mild Activity, Hi PS group scored worse than the Mod-Sev Activity, Low PS group with p=0.01. ns = not significant
DISCUSSION
Self-reported photosensitivity encompasses all skin-specific or systemic adverse reactions that the subject temporally relates to sun exposure. Self-reported photosensitivity is very common among CLE patients in the U.S. Approximately 68% of our subjects experience photosensitivity at a given time during the course of their disease. SCLE, LET, and ACLE appear to be the most photosensitive subtypes with the prevalence of self-reported photosensitivity being 88%, 80%, and 77% respectively. Even among the least photosensitive subtype, DLE, over 50% of subjects report experiencing photosensitivity. Among those with SLE with nonspecific skin manifestations, over 75% reported photosensitivity. Our results are similar to those of a U.S. population of SLE patients in which 73% reported experiencing photosensitivity via visual analogue scale14 and among a European CLE population that met SLE criteria, in which 63% of patients had observed photosensitivity39. Among CLE subtypes, our findings are congruent with reports that SCLE and LET patients are more likely to be photosensitive by history and by positive provocative phototest compared to DLE patients12,22,40.
We were able to classify subjects into a PS group, NOT PS group, and PS Suggestive group based on two simple clinical interview questions: “Have you been experiencing sensitivity to sunlight?” and “Do you have a history of photosensitivity?” The photosensitive group was comprised of subjects who reported a history of and current adverse reactions to sunlight. This group more frequently engaged in sun-protective behaviors and experienced lesions in sun-exposed areas. Further, the photosensitive group scored worse on the two photosensitivity items and PS scale of the modified Skindex-29+3. Our results suggest that simple interview questions are a reliable way of determining self-reported photosensitivity among LE subjects.
Both the PS group and PS Suggestive groups scored worse than the NOT PS group on the modified Skindex-29+3 photosensitivity items: worry about sun exposure, avoid outdoor behavior, and PS scale. Interestingly, only the behavior-related item, “I avoid outdoor activities because of my disease” showed a dose response, with the PS group scoring worse than the PS Suggestive group and even more so compared with the NOT PS group. These results suggest that items relating to behavior modification might be more sensitive predictors of significant photosensitivity in lupus.
We found that subjects reporting any symptoms of photosensitivity (PS and PS Suggestive groups) had greater cutaneous disease activity compared to subjects who denied ever experiencing photosensitivity. In support of this observation, we found that photosensitive subjects reported more impaired quality of life related to cutaneous symptoms compared to less photosensitive subjects with a similar level of cutaneous lupus activity. Among SLE populations, photosensitivity has been associated with both more severe systemic and more benign outcomes41–44. We are unaware, however, of any association between increased cutaneous lupus disease activity and photosensitivity in lupus. A relationship between UVR-induced lupus-specific skin lesions and cutaneous lupus activity is easily extrapolated, but it is less clear how non-specific photosensitivity reactions (ones excluding UVR-induced lupus-specific skin lesions) or sunlight-induced systemic lupus symptoms may relate to lasting cutaneous inflammation. Studies investigating mechanisms that underlie these general photosensitivity reactions within a CLE population to determine how they might contribute to cutaneous lupus-specific disease activity would be very interesting.
Patients with CLE that experience photosensitivity have a worse quality of life with respect to their daily functioning, symptoms, and emotions and the effect of photosensitivity appears to be independent of cutaneous lupus disease activity. Despite the effect of CLASI activity on symptoms and emotions, patients with more photosensitivity report greater impairment related to symptoms and emotions compared with less photosensitive patients with a similar degree of cutaneous lupus disease activity. The effect of photosensitivity on daily functioning is most striking, with highly photosensitive patients reporting significantly more impaired functioning compared to patients with more severe cutaneous lupus activity but less photosensitivity.
Prior studies have reported impaired quality of life among SLE patients with photosensitivity14, but we report on the impact of photosensitivity on quality of life in a primarily CLE population. This study contributes to a growing body of research suggesting that quality of life in cutaneous lupus is quite poor, and in fact, is as poor as in patients with common chronic medical conditions such as congestive heart failure and type 2 diabetes32. Photosensitivity is common among patients with CLE and is an important contributor to poor quality of life. Patients’ daily functioning is profoundly impacted not only by the cutaneous and systemic reactions to sun exposure but also by compliance with strict sun avoidance and sun protective behaviors.
As outlined in a recent editorial45, photoprotection for patients with lupus is of utmost importance. However, sun avoidance between 10 am and 3 pm and daily use of a hat and sun-protective clothing may not be easily adopted behaviors. Compliance with strict photoprotection may impair patients’ daily functioning. It is important for physicians to be aware of this when advising patients on photoprotection. Since photoprotection with sunscreens and clothing is difficult and sun avoidance can dramatically impact quality of life, future studies investigating the mechanisms that underlie self-reported photosensitivity in lupus are imperative.
One limitation of this study is that the subjects are patients treated at the medical dermatology clinic at the Hospital of the University of Pennsylvania, which is a referral-only center. As such, these patients might have more severe disease than cutaneous lupus patients that are treated by general dermatologists. Second, the cross-sectional nature of this study does not allow for examination of a causal relationship between photosensitivity and cutaneous disease activity or poor quality of life. Since we ask subjects to recall adverse reactions to sunlight, there could be an element of information bias that could artificially inflate the prevalence of self-reported photosensitivity in the sample. Lastly, patients with self-perceived photosensitivity might alter their behavior or worry more about sunlight that could confound the results regarding the effect of photosensitivity on quality of life.
CONCLUSION
Self-reported photosensitivity encompasses any adverse reaction that is identified by the patient and is felt to be related to sun exposure. Self-reported photosensitivity is very common among patients with CLE. Simple interview-style questions can be used to distinguish a population of patients who ascribe to sun-related adverse effects, have photodistributed skin lesions, and engage in sun protective behaviors, all of which confirm that the patient is photosensitive. Photosensitive patients can be further distinguished by the modified Skindex-29+ 3 items, of which the behavior-related item may be most sensitive. Photosensitivity is associated with worse cutaneous lupus activity and poor quality of life related to symptoms and emotions. Photosensitivity profoundly affects functioning such that the most photosensitive patients experience more impaired daily functioning compared to those with less photosensitivity but more severe cutaneous lupus disease activity.
Future studies should attempt to characterize the various phenotypes of self-reported photosensitivity. Photosensitivity surveys should incorporate behavioral items because they might be significant indicators of photosensitivity. Finally, the mechanisms underlying self-reported photosensitivity should be investigated to determine how they contribute to disease activity and to explore their use in the development of novel therapies that prevent or ameliorate sunlight-induced adverse symptoms among LE patients.
Table 3.
Mean CLASI activity scores among three photosensitivity groups
| N | Mean | SD | p | |
|---|---|---|---|---|
| NOT PS group | 40 | 4.6 | 5.6 | -- |
| PS Suggestive group | 23 | 10 | 11.7 | 0.072 |
| PS group | 91 | 9.2 | 9.5 | 0.023* |
there was a significant difference between the PS group and NOT PS group.
Acknowledgments
This material is based upon work supported by the National Institutes of Health, including NIH K24-AR 18 02207 (Werth), the Clinical and Translational Science Award (CTSA) TL1-RR-024133 (Foering), and by a Merit Review Grant from the Department of Veterans Affairs, Veteran Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development.
Abbreviations and Acronyms
- ACLE
Acute cutaneous lupus erythematosus
- ACR
American College of Rheumatology
- CCLE
Chronic cutaneous lupus erythematosus
- CLASI
Cutaneous lupus erythematosus disease area and severity index
- CLE
Cutaneous lupus erythematosus
- DLE
Discoid lupus erythematosus
- LE
lupus erythematosus
- LET
Tumid lupus erythematosus
- NOT PS group
Not photosensitive group
- PS Suggestive group
History of or new photosensitivity symptoms group
- PS scale
Photosensitivity scale
- PS group
Photosensitive group
- SCLE
Subacute cutaneous lupus erythematosus
- SLE
Systemic lupus erythematosus
- UVR
Ultraviolet radiation
Footnotes
The authors declare no conflict of interest.
This work was presented in a poster session at the Society for Investigative Dermatology annual meeting in May 2010.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Werth VP, Bashir M, Zhang W. Photosensitivity in rheumatic diseases. Journal of Investigative Dermatology Symposium Proceedings. 2004 Jan;9(1):57–63. doi: 10.1111/j.1087-0024.2004.00839.x. [DOI] [PubMed] [Google Scholar]
- 2.Lehmann P, Homey B. Clinic and pathophysiology of photosensitivity in lupus erythematosus. Autoimmunity Reviews. 2009 May;8(6):456–61. doi: 10.1016/j.autrev.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Kuhn A, Sonntag M, Richter-Hintz D, Oslislo C, Megahed M, Ruzicka T, Lehmann P. Phototesting in lupus erythematosus: A 15-year experience. J Am Acad Dermatol. 2001 Jul;45(1):86–95. doi: 10.1067/mjd.2001.114589. [DOI] [PubMed] [Google Scholar]
- 4.Nived O, Johansen PB, Sturfelt G. Standardized ultraviolet-A exposure provokes skin reaction in systemic lupus erythematosus. Lupus. 1993 Aug;2(4):247–50. doi: 10.1177/096120339300200407. [DOI] [PubMed] [Google Scholar]
- 5.Hasan T, Nyberg F, Stephansson E, Puska P, Hakkinen M, Sarna S, Ros AM, Ranki A. Photosensitivity in lupus erythematosus, UV photoprovocation results compared with history of photosensitivity and clinical findings. Br J Dermatol. 1997 May;136(5):699–705. [PubMed] [Google Scholar]
- 6.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 7.Lokitz ML, Billet S, Patel P, Kwon EJ, Sayre RM, Sullivan KE, Werth VP. Failure of physiologic doses of pure UVA or UVB to induce lesions in photosensitive cutaneous lupus erythematosus: Implications for phototesting. Photodermatol Photoimmunol Photomed. 2006;22(6):290–6. doi: 10.1111/j.1600-0781.2006.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doria A, Biasinutto C, Ghirardello A, Sartori E, Rondinone R, Piccoli A, Veller Fornasa C, Gambari PF. Photosensitivity in systemic lupus erythematosus: Laboratory testing of ARA/ACR definition. Lupus. 1996 Aug;5(4):263–8. doi: 10.1177/096120339600500404. [DOI] [PubMed] [Google Scholar]
- 9.Leenutaphong V, Boonchai W. Phototesting in oriental patients with lupus erythematosus. Photodermatol Photoimmunol Photomed. 1999 Feb;15(1):7–12. doi: 10.1111/j.1600-0781.1999.tb00045.x. [DOI] [PubMed] [Google Scholar]
- 10.Epstein JH, Tuffanelli, DuBois EL. Light sensitivity and lupus erythematosus. Arch Dermatol. 1965 May;91:483–5. doi: 10.1001/archderm.1965.01600110069013. [DOI] [PubMed] [Google Scholar]
- 11.Tuffanelli DL, DuBois EL. Cutaneous manifestations of systemic lupus erythematosus. Arch Dermatol. 1964 Oct;90:377–86. doi: 10.1001/archderm.1964.01600040005001. [DOI] [PubMed] [Google Scholar]
- 12.Sanders CJG, Van Weelden H, Kazzaz GAA, Sigurdsson V, Toonstra J, Bruijnzeel-Koomen CAFM. Photosensitivity in patients with lupus erythematosus: A clinical and photobiological study of 100 patients using a prolonged phototest protocol. Br J Dermatol. 2003;149(1):131–7. doi: 10.1046/j.1365-2133.2003.05379.x. [DOI] [PubMed] [Google Scholar]
- 13.Kuhn A, Beissert S. Photosensitivity in lupus erythematosus. Autoimmunity. 2005 Nov;38(7):519–29. doi: 10.1080/08916930500285626. [DOI] [PubMed] [Google Scholar]
- 14.Wysenbeek AJ, Block DA, Fries JF. Prevalence and expression of photosensitivity in systemic lupus erythematosus. Ann Rheum Dis. 1989 Jun;48(6):461–3. doi: 10.1136/ard.48.6.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sontheimer RD, Thomas JR, Gilliam JN. Subacute cutaneous lupus erythematosus: A cutaneous marker for a distinct lupus erythematosus subset. Arch Dermatol. 1979 Dec;115(12):1409–15. [PubMed] [Google Scholar]
- 16.Sontheimer RD, Maddison PJ, Reichlin M, Jordon RE, Stastny P, Gilliam JN. Serologic and HLA associations in subacute cutaneous lupus erythematosus, a clinical subset of lupus erythematosus. Ann Intern Med. 1982 Nov;97(5):664–71. doi: 10.7326/0003-4819-97-5-664. [DOI] [PubMed] [Google Scholar]
- 17.Herrero C, Bielsa I, Font J, Lozano F, Ercilla G, Lecha M, Ingelmo M, Mascaro JM. Subacute cutaneous lupus erythematosus: Clinicopathologic findings in thirteen cases. J Am Acad Dermatol. 1988 Dec;19(6):1057–62. doi: 10.1016/s0190-9622(88)70272-8. [DOI] [PubMed] [Google Scholar]
- 18.Callen JP, Klein J. Subacute cutaneous lupus erythematosus. clinical, serologic, immunogenetic, and therapeutic considerations in seventy-two patients. Arthritis Rheum. 1988 Aug;31(8):1007–13. doi: 10.1002/art.1780310811. [DOI] [PubMed] [Google Scholar]
- 19.Callen JP. Chronic cutaneous lupus erythematosus. clinical, laboratory, therapeutic, and prognostic examination of 62 patients. Arch Dermatol. 1982 Jun;118(6):412–6. doi: 10.1001/archderm.118.6.412. [DOI] [PubMed] [Google Scholar]
- 20.Callen JP. Photosensitivity in collagen vascular diseases. Semin Cutan Med Surg. 1999 Dec;18(4):293–6. doi: 10.1016/s1085-5629(99)80028-5. [DOI] [PubMed] [Google Scholar]
- 21.Sontheimer RD, Provost TT. Lupus erythematosus. In: Sontheimer RD, Provost TT, editors. Cutaneous manifestations of rheumatic diseases. Baltimore: Williams and Wilkins; 1996. [Google Scholar]
- 22.Lehmann P, Holzle E, Kind P, Goerz G, Plewig G. Experimental reproduction of skin lesions in lupus erythematosus by UVA and UVB radiation. J Am Acad Dermatol. 1990 Feb;22(2 Pt 1):181–7. doi: 10.1016/0190-9622(90)70020-i. [DOI] [PubMed] [Google Scholar]
- 23.Kind P, Lehmann P, Plewig G. Phototesting in lupus erythematosus. J Invest Dermatol. 1993 Jan;100(1):53S–7S. doi: 10.1111/1523-1747.ep12355594. [DOI] [PubMed] [Google Scholar]
- 24.Walchner M, Messer G, Kind P. Phototesting and photoprotection in LE. Lupus. 1997;6(2):167–74. doi: 10.1177/096120339700600212. [DOI] [PubMed] [Google Scholar]
- 25.Beutner EH, Blaszczyk M, Jablonska S, Chorzelski TP, Kumar V, Wolska H. Studies on criteria of the european academy of dermatology and venerology for the classification of cutaneous lupus erythematosus. I. selection of clinical groups and study factors. Int J Dermatol. 1991 Jun;30(6):411–7. doi: 10.1111/j.1365-4362.1991.tb03896.x. [DOI] [PubMed] [Google Scholar]
- 26.Kuhn A, Sonntag M, Richter-Hintz D, Oslislo C, Megahed M, Ruzicka T, Lehmann P. Phototesting in lupus erythematosus tumidus--review of 60 patients. Photochem Photobiol. 2001 May;73(5):532–6. doi: 10.1562/0031-8655(2001)073<0532:piletr>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 27.Wolska H, Blaszczyk M, Jablonska S. Phototests in patients with various forms of lupus erythematosus. Int J Dermatol. 1989 Mar;28(2):98–103. doi: 10.1111/j.1365-4362.1989.tb01327.x. [DOI] [PubMed] [Google Scholar]
- 28.Gilliam JN, Sontheimer RD. Subacute cutaneous lupus erythematosus. Clin Rheum Dis. 1982 Aug;8(2):343–52. [PubMed] [Google Scholar]
- 29.Chren MM, Lasek RJ, Flocke SA, Zyzanski SJ. Improved discriminative and evaluative capability of a refined version of skindex, a quality-of-life instrument for patients with skin diseases. Arch Dermatol. 1997 Nov;133(11):1433–40. [PubMed] [Google Scholar]
- 30.Chren MM, Lasek RJ, Quinn LM, Mostow EN, Zyzanski SJ. Skindex, a quality-of-life measure for patients with skin disease: Reliability, validity, and responsiveness. J Invest Dermatol. 1996 Nov;107(5):707–13. doi: 10.1111/1523-1747.ep12365600. [DOI] [PubMed] [Google Scholar]
- 31.Chren MM, Lasek RJ, Quinn LM, Covinsky KE. Convergent and discriminant validity of a generic and a disease-specific instrument to measure quality of life in patients with skin disease. J Invest Dermatol. 1997 Jan;108(1):103–7. doi: 10.1111/1523-1747.ep12285650. [DOI] [PubMed] [Google Scholar]
- 32.Klein R, Moghadam-Kia S, Taylor L, Coley C, Okawa J, LoMonico J, Chren MM, Werth VP. Quality of life in cutaneous lupus erythematosus. J Am Acad Dermatol. 2010 doi: 10.1016/j.jaad.2010.02.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moghadam-Kia S, Chilek K, Gaines E, Costner M, Rose ME, Okawa J, Werth VP. Cross-sectional analysis of a collaborative web-based database for lupus erythematosus-associated skin lesions: Prospective enrollment of 114 patients. Arch Dermatol. 2009 Mar;145(3):255–60. doi: 10.1001/archdermatol.2008.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonilla-Martinez ZL, Albrecht J, Troxel AB, Taylor L, Okawa J, Dulay S, Werth VP. The cutaneous lupus erythematosus disease area and severity index: A responsive instrument to measure activity and damage in patients with cutaneous lupus erythematosus. Arch Dermatol. 2008 Feb;144(2):173–80. doi: 10.1001/archderm.144.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Albrecht J, Werth VP. Development of the CLASI as an outcome instrument for cutaneous lupus erythematosus. Dermatol Ther. 2007 Mar-Apr;20(2):93–101. doi: 10.1111/j.1529-8019.2007.00117.x. [DOI] [PubMed] [Google Scholar]
- 36.Albrecht J, Taylor L, Berlin JA, Dulay S, Ang G, Fakharzadeh S, Kantor J, Kim E, Militello G, McGinnis K, Richardson S, Treat J, Vittorio C, Van Voorhees A, Werth VP. The CLASI (cutaneous lupus erythematosus disease area and severity index): An outcome instrument for cutaneous lupus erythematosus. J Invest Dermatol. 2005 Nov;125(5):889–94. doi: 10.1111/j.0022-202X.2005.23889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krathen MS, Dunham J, Gaines E, Junkins-Hopkins J, Kim E, Kolasinski SL, Kovarik C, Kwan-Morley J, Okawa J, Propert K, Rogers N, Rose M, Thomas P, Troxel AB, Van Voorhees A, Feldt JV, Weber AL, Werth VP. The cutaneous lupus erythematosus disease activity and severity index: Expansion for rheumatology and dermatology. Arthritis Rheum. 2008 Mar 15;59(3):338–44. doi: 10.1002/art.23319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klein R, Moghadam-Kia S, LoMonico J, Okawa J, Coley Ch, Taylor L, Troxel AB, Werth VP. Development of the CLASI as a tool to measure disease severity and responsiveness to therapy in cutaneous lupus erythematosus. Arch Dermatol. doi: 10.1001/archdermatol.2010.435. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yell JA, Mbuagbaw J, Burge SM. Cutaneous manifestations of systemic lupus erythematosus. Br J Dermatol. 1996 Sep;135(3):355–62. [PubMed] [Google Scholar]
- 40.Vera-Recabarren MA, Garcia-Carrasco M, Ramos-Casals M, Herrero C. Comparative analysis of subacute cutaneous lupus erythematosus and chronic cutaneous lupus erythematosus: Clinical and immunological study of 270 patients. Br J Dermatol. 2010;162(1):91–101. doi: 10.1111/j.1365-2133.2009.09472.x. [DOI] [PubMed] [Google Scholar]
- 41.Parodi A, Massone C, Cacciapuoti M, Aragone MG, Bondavalli P, Cattarini G, Rebora A. Measuring the activity of the disease in patients with cutaneous lupus erythematosus. Br J Dermatol. 2000 Mar;142(3):457–60. doi: 10.1046/j.1365-2133.2000.03356.x. [DOI] [PubMed] [Google Scholar]
- 42.Leong KP, Chong EY, Kong KO, Chan SP, Thong BY, Lian TY, Chng HH, Koh ET, Teh CL, Lau TC, Law WG, Cheng YK, Badsha H, Chew LC, Yong WH, Howe HS Tan Tock Seng Hospital (TTSH) Lupus Study Group. Discordant assessment of lupus activity between patients and their physicians: The singapore experience. Lupus. 2010 Jan;19(1):100–6. doi: 10.1177/0961203309345748. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez LA, Pons-Estel GJ, Zhang J, Vila LM, Reveille JD, Alarcon GS LUMINA study group. Time to neuropsychiatric damage occurrence in LUMINA (LXVI): A multi-ethnic lupus cohort. Lupus. 2009 Aug;18(9):822–30. doi: 10.1177/0961203309104392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bertoli AM, Vila LM, Apte M, Fessler BJ, Bastian HM, Reveille JD, Alarcon GS LUMINA Study Group. Systemic lupus erythematosus in a multiethnic US cohort LUMINA XLVIII: Factors predictive of pulmonary damage. Lupus. 2007;16(6):410–7. doi: 10.1177/0961203307079042. [DOI] [PubMed] [Google Scholar]
- 45.Obermoser G, Zelger B. Triple need for photoprotection in lupus erythematosus. Lupus. 2008;17(6):525–7. doi: 10.1177/0961203308089440. [DOI] [PubMed] [Google Scholar]