Fig. 4.
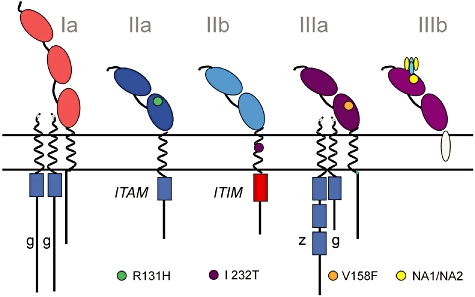
Single nucleotide polymorphisms (SNPs) in various human Fcγ receptors (FcγR). Different types of single nucleotide polymorphisms (SNPs) are known to have functional implications related to the affinity of the FcγR to IgG binding and/or signalling capacity. With respect to the SNP in the intramembranous domain of the inhibitory FcγRIIb, the infrequent FcγRIIb-232T variant is believed to give a stronger negative signal via the immunoreceptor tyrosine-based inhibitory motif (ITIM). FcγRIIa-H131 binds human IgG2, whereas FcγRIIa-R131 does not. In FcγRIIIa, FcγRIIIa-V158 has a higher affinity for IgG1 and IgG3 than FcγRIIIa-F158. Although some of the FcγRs do not contain the immunoreceptor tyrosine-based activating motif (ITAM), these receptors are associated with other proteins for signalling. Allelic variation in FcγRIIIb is composed of differences in four amino acids, referred to as human neutrophil antigen 1 [HNA1a (NA1) and HNA1b (NA2)]; FcγRIIIb-HNA1a internalizes IgG1- or IgG3-opsonized particles more efficiently than does FcγRIIIb-HNA1b.
