Abstract
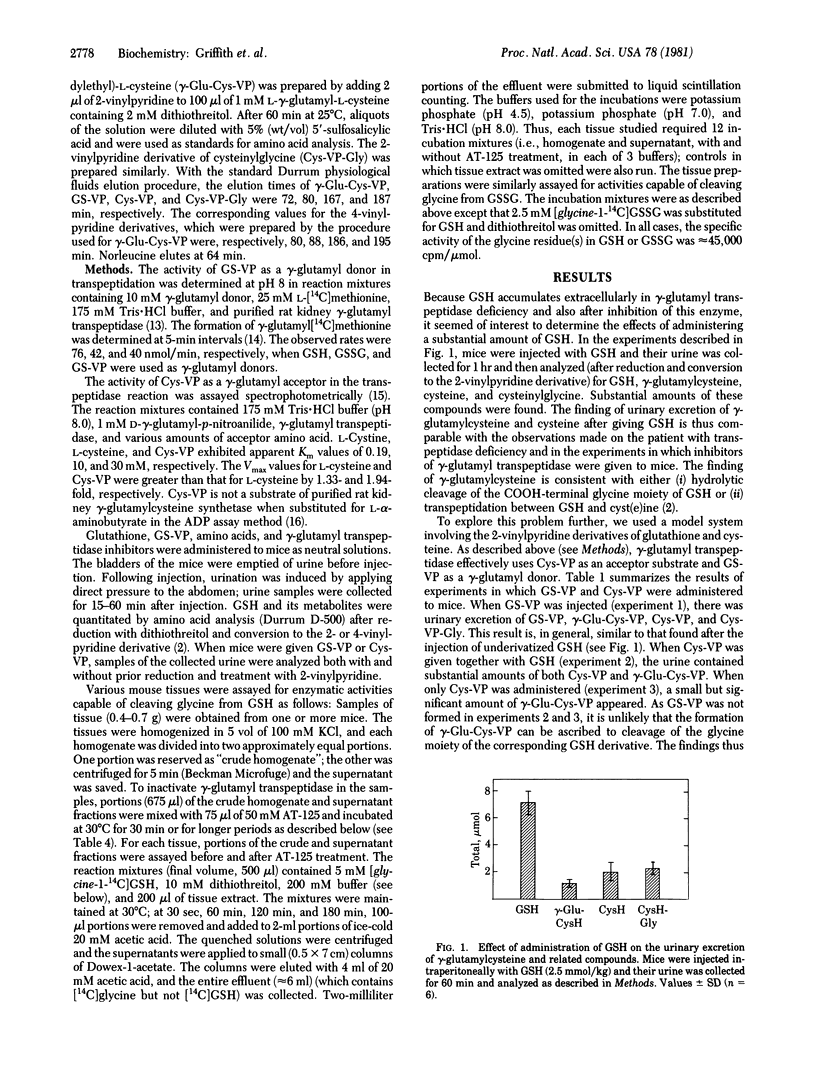
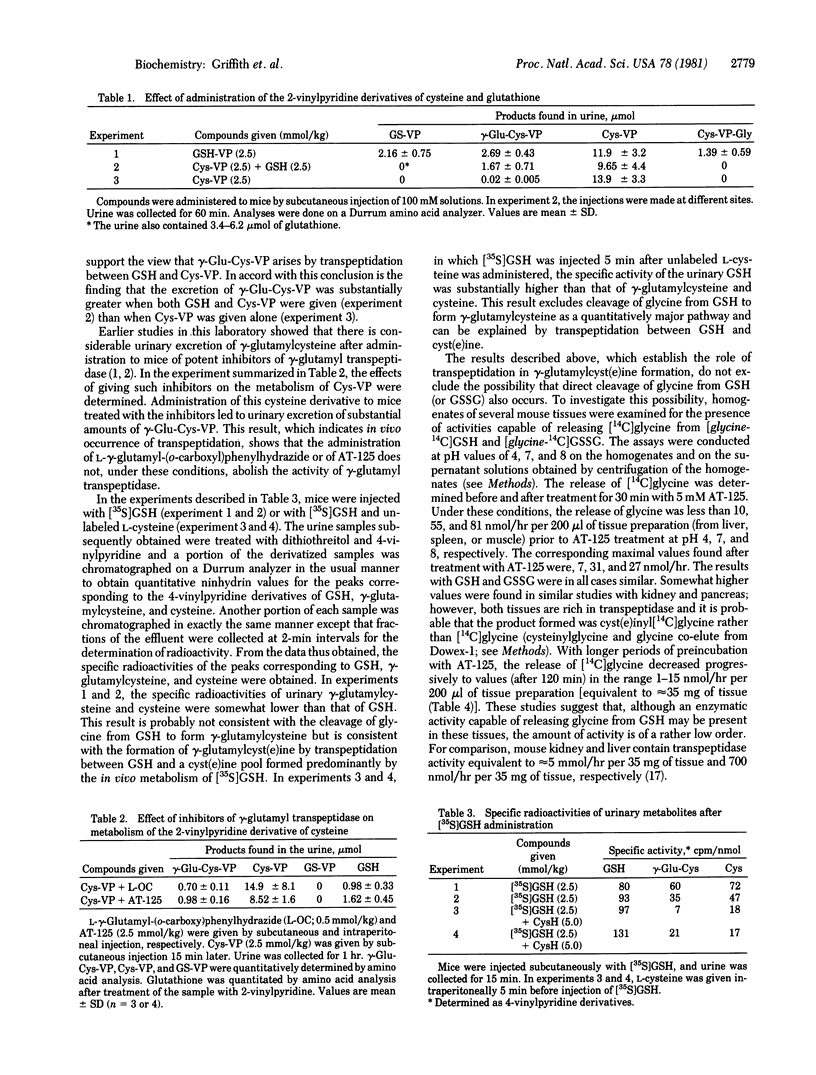
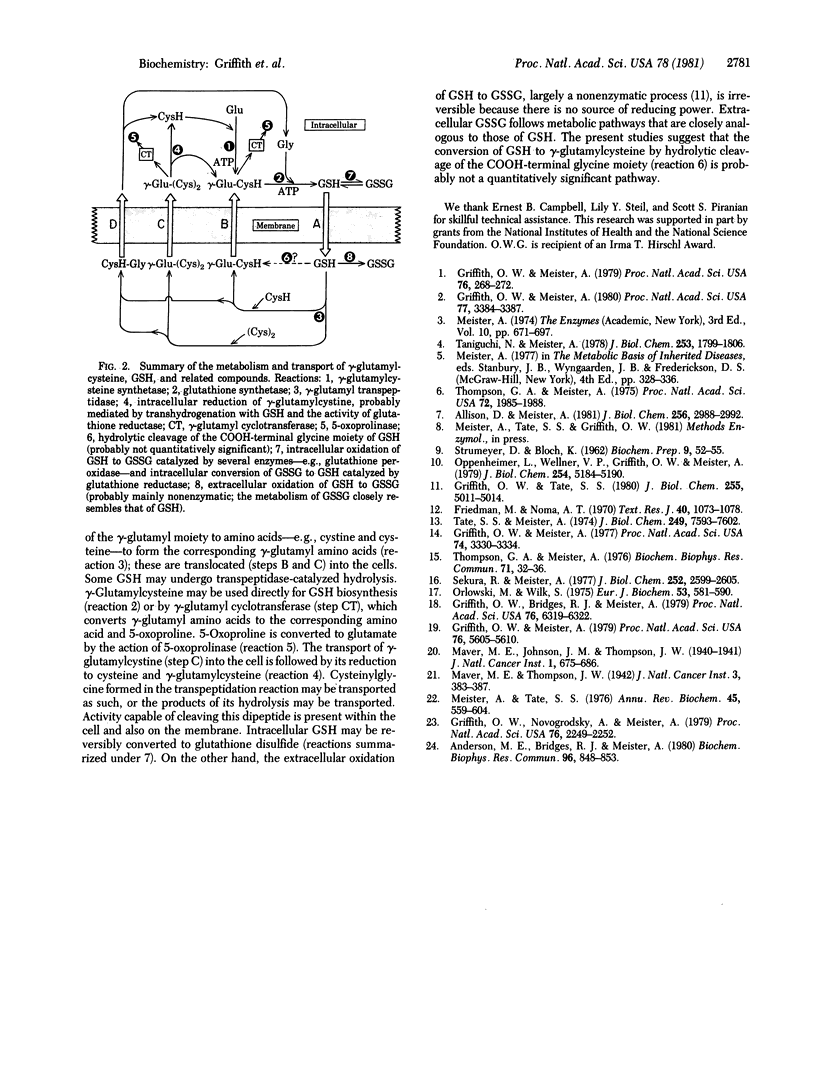
These studies indicate that gamma-glutamylcyst(e)ine, found in the urine of a patient with gamma-glutamyl transpeptidase deficiency and also in the urine of experimental animals injected with glutathione or with inhibitors of gamma-glutamyl transpeptidase, is formed by the action of gamma-glutamyltranspeptidase. The evidence demonstrates that transpeptidation between glutathione and cystine occurs in vivo and also that this reaction constitutes a significant physiological function of the enzyme. The appearance of large amounts of gamma-glutamylcyst(e)ine in the urine seems to reflect an inhibitory effect of glutathione on the transport of gamma-glutamylcyst(e)ine into cells. The findings also indicate that conversion of glutathione to gamma-glutamylcysteine by hydrolytic cleavage of the COOH-terminal glycine moiety of glutathione (or analogous cleavage of glutathione disulfide) is not a quantitatively significant pathway. The results reported here show that gamma-glutamyl transpeptidase activity is not completely absent in a patient found to have a deficiency of this enzyme and that the activity of the enzyme is not abolished in experimental animals treated with potent gamma-glutamyl transpeptidase inhibitors.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison R. D., Meister A. Evidence that transpeptidation is a significant function of gamma-glutamyl transpeptidase. J Biol Chem. 1981 Mar 25;256(6):2988–2992. [PubMed] [Google Scholar]
- Anderson M. E., Bridges R. J., Meister A. Direct evidence for inter-organ transport of glutathione and that the non-filtration renal mechanism for glutathione utilization involves gamma-glutamyl transpeptidase. Biochem Biophys Res Commun. 1980 Sep 30;96(2):848–853. doi: 10.1016/0006-291x(80)91433-3. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Bridges R. J., Meister A. Transport of gamma-glutamyl amino acids: role of glutathione and gamma-glutamyl transpeptidase. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6319–6322. doi: 10.1073/pnas.76.12.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Excretion of cysteine and gamma-glutamylcysteine moieties in human and experimental animal gamma-glutamyl transpeptidase deficiency. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3384–3387. doi: 10.1073/pnas.77.6.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Glutathione: interorgan translocation, turnover, and metabolism. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5606–5610. doi: 10.1073/pnas.76.11.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Selective inhibition of gamma-glutamyl-cycle enzymes by substrate analogs. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3330–3334. doi: 10.1073/pnas.74.8.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Translocation of intracellular glutathione to membrane-bound gamma-glutamyl transpeptidase as a discrete step in the gamma-glutamyl cycle: glutathionuria after inhibition of transpeptidase. Proc Natl Acad Sci U S A. 1979 Jan;76(1):268–272. doi: 10.1073/pnas.76.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W., Novogrodsky A., Meister A. Translocation of glutathione from lymphoid cells that have markedly different gamma-glutamyl transpeptidase activities. Proc Natl Acad Sci U S A. 1979 May;76(5):2249–2252. doi: 10.1073/pnas.76.5.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W., Tate S. S. The apparent glutathione oxidase activity of gamma-glutamyl transpeptidase. Chemical mechanism. J Biol Chem. 1980 Jun 10;255(11):5011–5014. [PubMed] [Google Scholar]
- Meister A., Tate S. S. Glutathione and related gamma-glutamyl compounds: biosynthesis and utilization. Annu Rev Biochem. 1976;45:559–604. doi: 10.1146/annurev.bi.45.070176.003015. [DOI] [PubMed] [Google Scholar]
- Oppenheimer L., Wellner V. P., Griffith O. W., Meister A. Glutathione synthetase. Purification from rat kidney and mapping of the substrate binding sites. J Biol Chem. 1979 Jun 25;254(12):5184–5190. [PubMed] [Google Scholar]
- Orlowski M., Wilk S. Intermediates of the gamma-glutamyl cycle in mouse tissues. Influence of administration of amino acids on pyrrolidone carboxylate and gamma-glutamyl amino acids. Eur J Biochem. 1975 May 6;53(2):581–590. doi: 10.1111/j.1432-1033.1975.tb04101.x. [DOI] [PubMed] [Google Scholar]
- Sekura R., Meister A. gamma-Glutamylcysteine synthetase. Further purification, "half of the sites" reactivity, subunits, and specificity. J Biol Chem. 1977 Apr 25;252(8):2599–2605. [PubMed] [Google Scholar]
- Taniguchi N., Meister A. gamma-Glutamyl cyclotransferase from rat kidney. Sulfhydryl groups and isolation of a stable form of the enzyme. J Biol Chem. 1978 Mar 25;253(6):1799–1806. [PubMed] [Google Scholar]
- Tate S. S., Meister A. Interaction of gamma-glutamyl transpeptidase with amino acids, dipeptides, and derivatives and analogs of glutathione. J Biol Chem. 1974 Dec 10;249(23):7593–7602. [PubMed] [Google Scholar]
- Thompson G. A., Meister A. Hydrolysis and transfer reactions catalyzed by gamma-glutamyl transpeptidase; evidence for separate substrate sites and for high affinity of L-cystine. Biochem Biophys Res Commun. 1976 Jul 12;71(1):32–36. doi: 10.1016/0006-291x(76)90245-x. [DOI] [PubMed] [Google Scholar]
- Thompson G. A., Meister A. Utilization of L-cystine by the gamma-glutamyl transpeptidase-gamma-glutamyl cyclotransferase pathway. Proc Natl Acad Sci U S A. 1975 Jun;72(6):1985–1988. doi: 10.1073/pnas.72.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


