Abstract
Traditional microbiological techniques are used to provide reliable data on the rate and extent of kill for a range of biocides. However, such techniques provide very limited data regarding the initial rate of kill of fast-acting biocides over very short time domains. This study describes the application of a recombinant strain of Escherichia coli expressing the Photorhabdus luminescens lux operon as a whole-cell biosensor. Light emission is linked directly to bacterial metabolism; therefore, by monitoring light output, the impact of fast-acting biocides can be assessed. Electrochemically activated solutions (ECASs), bleach, Virkon, and ethanol were assessed at three concentrations (1%, 10%, 80%) in the presence of organic soiling. Over a 2-s time course, 80% ECAS produced the greatest reduction in light output in the absence of organic load but was strongly inhibited by its presence. Eighty percent ethanol outperformed all tested biocides in the presence of organic soil. Bleach and Virkon produced similar reductions in bioluminescence at matched concentrations within the time course of the assay. It was also demonstrated that the assay can be used to rapidly assess the impact of organic soiling. The use of bioluminescent bacteria as whole-cell bioreporters allows assessment of the relative efficacies of fast-acting biocides within milliseconds of application. The assay can be used to investigate activity over short or extended time domains to confirm complete metabolic inhibition of the bioreporter. Moreover, the assay may enable further elucidation of their mechanism of action by allowing the investigation of activity over time domains precluded by traditional microbiology.
INTRODUCTION
The efficacy of electrochemically activated solutions (ECASs) as fast-acting bactericidal and sporicidal biocides active against a range of organisms has been previously reported (26). It was noted that traditional microbiological methods were limiting in terms of elucidating the efficacy of biocides with a rapid mode of action, particularly during the early stages of kill. Loshon et al. (18) had similar difficulties when assessing the kill rate of a related biocide against spores of Bacillus subtilis. Traditional recovery count methods are limited by the frequency of the sampling time and the duration required to transfer the sample to a neutralizing diluent. This limitation is exacerbated when examining fast-acting biocides, where the time required for removal of a sample and adequate mixing with a neutralizer may be significant in terms of the kill kinetics. Few studies directly compare biocides using suitable methods, and the availability of simple, nonspecific tests is of benefit to users in selecting a biocide appropriate to their needs (31). This study describes a real-time technique, utilizing bioluminescent bacteria as whole-cell bioreporters of the very early stage of kill, for a range of fast-acting biocides under clean and dirty conditions.
Bioluminescence occurs throughout nature in many species, the most abundant of which are bacteria (23). Most bioluminescent bacteria have been classified into the genera Vibrio and Photobacterium (both marine dwelling), along with a single terrestrial example, Photorhabdus luminescens (24). Bioluminescence provides an ideal bioreporter system that produces a physical rather than chemical signal, thus avoiding accumulation of compounds that may lead to toxicity or instability, as has been shown with green fluorescent protein expression in Escherichia coli (29). Light emission can be accurately measured with a high level of sensitivity (using an appropriate apparatus) in a nondestructive fashion and in real time (3).
It has been shown that the Vibrio fischeri lux operon can be inserted into and expressed in E. coli via a transposon to generate a bioluminescent phenotype that did not require the addition of exogenous substrate (12). The lux genes required for bacterial bioluminescence are arranged in a single operon, luxCDABE. Since the operon encodes not only the luciferase (luxAB) but also the enzyme complex responsible for the synthesis of the luciferin substrate (luxCDE), all that is needed to produce light in a recombinant aerobic bacterium is the expression of the lux operon (14). An appropriate luciferase system must be utilized to produce a stable bacterial reporter. The luciferase from V. fischeri is unstable in E. coli at temperatures in excess of 30°C, and that of Vibrio harveyi is unstable at temperatures in excess of 37°C. The luciferase of Photorhabdus luminescens was found to be stable in E. coli at up to 42°C (14), making it well suited to the study of human pathogens. The reaction is dependent upon the oxidation of reduced flavin mononucleotide (FMNH2), part of the electron transport system. Therefore, in a lux-expressing bacterial cell where FMNH2 is constantly supplied, in the presence of adequate O2 levels, bioluminescence remains at a constant level (23). The coupling of bioluminescence to bacterial metabolic activity has afforded a unique means of studying the effect of antimicrobial compounds on bacteria in real time. The novel antimicrobial linezolid has been tested on Streptococcus pneumoniae expressing the P. luminescens lux operon. Bioluminescence point readings and recovery counts were performed hourly over 12 h and showed that bioluminescence gave an earlier indicator of cellular recovery than counting techniques (1). The same strain was used in a study of the efficacy of the fluoroquinolone antibiotic gemifloxacin, and light output was found to correlate with optical density measurements and viable counts during exponential growth (2). Other fluoroquinolones, including moxifloxacin and ciprofloxacin, have been used to challenge bioluminescent E. coli (27) and Pseudomonas aeruginosa (20), the latter in a continuous perfusion model of biofilm growth. Bioluminescent bacteria have also been used as an in vitro wound model for testing antimicrobial wound dressings (32). In all cases, expression of lux was shown to be a sensitive, real-time reporter of antimicrobial efficacy. Bacterial bioluminescence has also been utilized to assess the susceptibility of bacteria to biocides. Dhir and Dodd (10) used bioluminescent Salmonella enterica serovar Enteritidis to assess the susceptibility of the organism to phenol, chlorhexidine digluconate, and Virkon and acknowledged that bioluminescence could be used with confidence in circumstances where viable counting was impractical. Choi and Gu (8) used bioluminescent E. coli to assess membrane-damaging biocides such as phenol and ethanol, monitoring luminescence at 15-min intervals. It was concluded that bioluminescence may be used to distinguish the mechanism of action of biocides.
In addition to bacterial bioluminescence, ATP bioluminescence assays are commonly used to detect bacterial survival following decontamination treatments and are considered a rapid, simple approach to monitoring surface sanitation (17). Such approaches provide endpoint data following the application of a biocide and require biocide neutralization prior to detection and the addition of exogenous substrates. Our study uses self-bioluminescent bacteria as real-time reporters of the impact of fast-acting biocides at the point of application.
The main objectives of this study were to determine whether a bioluminescent reporter strain could be used as a whole-cell bioreporter to compare the efficacy of a range of fast-acting biocides and the impact of organic loading on that efficacy. E. coli Nissle 1917 was selected for this study due to the inherent safety of its probiotic nature, coupled with rapid growth on complex media. Following transformation, it proved to be a bright, stable, and consistent bioluminescent reporter, allowing the full dynamic range of the test luminometric equipment to be utilized. The ability to monitor the effect of fast-acting biocides on a bioluminescent reporter in real time would allow simple direct comparisons of the efficacy of biocides, the determination of optimum working concentrations, and the direct effect of interfering substances (organic loading) to be assessed.
MATERIALS AND METHODS
Construction of bioluminescent reporter strain.
E. coli Nissle 1917 was transformed with the recombinant plasmid pGLITE (25), encoding the P. luminescens lux operon from plasmid pLITE27 (19) inserted into the broad-host-range plasmid PBBR1MCS-2. Plasmid DNA was extracted from E. coli DH5α/pGLITE using a Wizard Plus SV Minipreps DNA purification system (Promega, Southampton, United Kingdom). E. coli Nissle 1917 (Ardeypharm GmbH, Germany) was cultured on LB agar plates (tryptone, 10 g liter−1; yeast extract, 5 g liter−1; NaCl, 5 g liter−1; bacteriological agar, 15 g liter−1) at 37°C. A single, isolated colony was transferred aseptically to 10 ml LB broth (tryptone, 10 g liter−1; yeast extract, 5 g liter−1; NaCl, 5 g liter−1) and grown in an orbital shaker at 37°C and 200 rpm for 18 h. One milliliter of this was transferred aseptically to an Erlenmeyer flask containing 200 ml fresh, prewarmed LB broth and grown at 37°C and 250 rpm until an optical density at 600 nm (OD600) of 0.5 was achieved. The cells were chilled on ice for 15 min, before being transferred to sterile 50-ml Falcon tubes and centrifuged for 20 min at 3,000 × g at 4°C (ALC PK121R; Jouan, Milan, Italy). The supernatant from each tube was discarded and cells were resuspended in 50 ml ice-cold 10% glycerol. The cells were then washed and resuspended in 25 ml, 5 ml, and, finally, 100 μl ice-cold 10% glycerol. Forty microliters of cell suspension was transferred to a chilled sterile Eppendorf tube and incubated on ice with 2 μl plasmid DNA for 1 min. The mix was transferred to a 2-mm electroporation cuvette (Bio-Rad Laboratories Ltd., Hemel Hempstead, United Kingdom) and pulsed once in a Bio-Rad GenePulser Xcell at 3 kV with a 25-μF capacitor and 200-Ω parallel resistor. Immediately following the pulse, 1 ml room temperature SOC resuscitation medium (4) (2.0 g tryptone, 0.5 g yeast extract, 0.2 ml 5 M NaCl, 0.25 ml 1 M KCl dissolved in 90 ml water, pH adjusted to 7.0, and then the volume was brought to 100 ml and autoclaved and 1.0 ml sterile 1 M MgCl2, 1.0 ml sterile 1 M MgSO4, and 2 ml filter-sterilized 1 M glucose were added) was added to the cuvette. The contents of the cuvette were transferred to a sterile Eppendorf tube and incubated at 37°C for 1 h with shaking at 225 rpm. Cells were diluted into sterile Ringer's solution and spread plated onto LB agar supplemented with 10 mg liter−1 kanamycin (Sigma-Aldrich Company Ltd., Poole, United Kingdom). Plates were checked for transformants after 24 and 48 h using a high-resolution photon-counting system (HRPCS-3; Photek, St Leonards on Sea, United Kingdom). Transformants were subcultured on LB agar supplemented with 10 mg liter−1 kanamycin.
Preparation of inoculum.
E. coli Nissle 1917/pGLITE was grown for 18 h in nutrient broth supplemented with 10 mg liter−1 kanamycin. This was subcultured 1:1,000 into 100 ml fresh prewarmed nutrient broth supplemented with 10 mg liter−1 kanamycin and grown at 37°C with shaking at 250 rpm until an OD600 of 0.5 was achieved. The cells were then centrifuged for 20 min at 3,000 × g, washed twice in an equal volume of sterile Ringer's solution, and then resuspended in 10 ml sterile Ringer's solution.
Preparation of biocides.
Bleach was prepared by diluting a commercial sodium hypochlorite solution (Pattersons bleach; Pattersons Ltd., Bristol, United Kingdom) in deionized water. Ethanol was prepared similarly by diluting absolute ethanol (Fisher Scientific, Loughborough, United Kingdom). Virkon (Antec International, Sudbury, United Kingdom) was prepared from 1% (wt/vol) powder in deionized water, according to the manufacturer's instructions, and further diluted in deionized water to the concentrations used in the assay. ECAS was generated according to the manufacturer's instructions (Purest Solutions Ltd., London, United Kingdom) with a pH of 2.2 to 2.4 and a redox potential of 1,170 to 1,180 mV and diluted in deionized water where required.
Luminometric assay.
Thirty microliters of inoculum was added to a clean borosilicate glass tube (12 by 75 mm; Fisher Scientific, Loughborough, United Kingdom) and loaded into a Sirius luminometer (Berthold Detection Systems, Pforzheim, Germany). In addition, 30 μl of fetal bovine serum (FBS), 15 μl of FBS and 15 μl of Ringer's solution, or 30 μl of Ringer's solution alone was added to represent 10%, 5%, and 0% organic soiling, respectively. The automated protocol included a 10-s delay to allow light levels to equilibrate. Bacterial bioluminescence was recorded every 0.2 s for 15 s prior to automated injection of 240 μl of biocide (diluted where appropriate), followed by a further 105 s of data collection. Control light levels were established by replacing the biocide with Ringer's solution, and background light levels were established by replacing the inoculum with Ringer's solution.
RESULTS
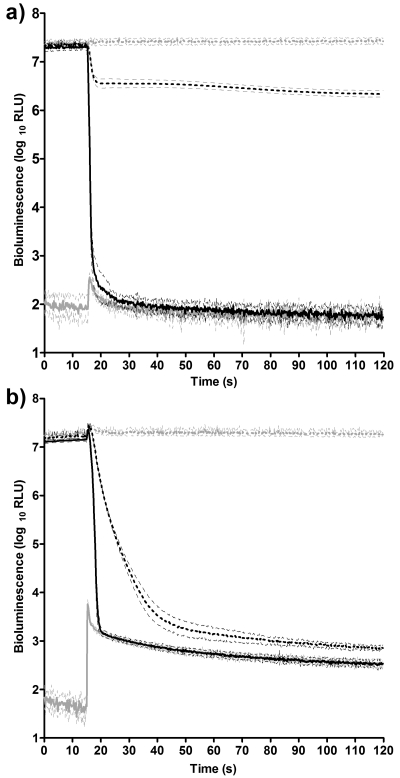
Preparation of the inoculum as described resulted in a culture with levels of bioluminescence approaching the upper limit of the tube luminometer, affording a full 5 decades of dynamic range. Direct comparisons of rates of reduction can be made over this range; however, a number of important factors must be considered. When considering log10 reductions in viable survivors, care must be taken to ensure a uniform initial inoculum in order to make valid comparisons. Similarly, when considering log10 reductions in bioluminescence, a uniform initial light output is a prerequisite for direct comparability. Figure 1a shows the effects of different concentrations of ethanol on bacterial bioluminescence in the presence of 10% FBS. Preinjection bioluminescence was consistent between treated and control populations. The injection of 1% ethanol had no measurable effect on light emission, which remained unchanged from the control (data not shown). Upon exposure to 10% and 80% ethanol, there were clear differences in the rate and extent of reduction of bioluminescence. When measuring background bioluminescence (in the absence of inoculum), there was a small, transient increase in light detected immediately following the injection of ethanol. This reduced back to preinjection levels within 10 s. Figure 1b shows the effects of different concentrations of Virkon on bacterial bioluminescence, also in the presence of 10% FBS. Preinjection bioluminescence was again consistent: 1% Virkon produced no measurable difference compared to the control (data not shown), and 10% and 80% clearly reduced bioluminescence. However, when measuring background bioluminescence, following the injection of Virkon, background light levels increased over 100-fold and remained 10-fold higher than the preinjection values for the remainder of the assay. This luminescent emission from the reagent blank was also observed to a greater extent with bleach and a lesser extent with ECAS (data not shown). In all cases, light emission from the reagent blank following injection of disinfectant was greatest using 80% biocide and 10% FBS. This suggests a light-bearing reaction between some of the tested biocides and FBS. This luminescent emission from the organic load significantly reduced the dynamic range of the instrument. Light output from a population, which is decreasing rapidly, can become conflated with that from a strongly emitting reagent blank. This is shown clearly in Fig. 1b, where the rate of decrease in light output from the population treated with 80% Virkon changes significantly at the point where the kill curve intercepts the background curve. Therefore, endpoint comparisons between biocides under conditions where light levels from the reagent blank would interfere with the kill curve are better served by traditional microbiology. However, valid comparisons can still be made during the very early stages of treatment, before background bioluminescence is reached. It has already been demonstrated that fast-acting biocides like ECAS can achieve a total kill of viable vegetative bacteria within 10 s of treatment (26, 30). These time domains can be investigated reliably only in real time, by using the target bacteria as whole-cell bioreporters. The Sirius luminometer was selected as a detector as it can report light levels at 0.2-s time intervals, allowing up to 11 measurements to be taken over the first 2 s of treatment.
Fig. 1.
Efficacy of ethanol (a) or Virkon (b) on light output from E. coli Nissle 1917/pGLITE at 1% (broken grey line), 10% (broken black line), and 80% (solid black line) and background bioluminescence from reagent blank (solid grey line). Light output is measured in RLU ± SD (n = 12).
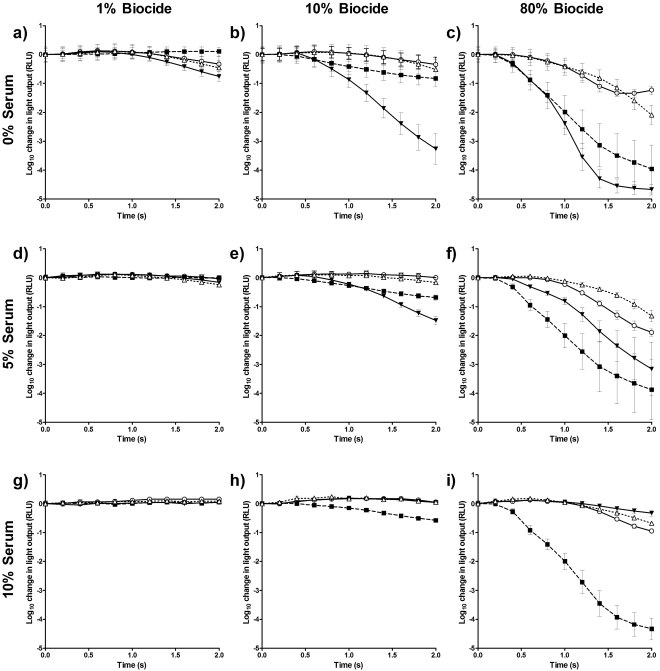
Figure 2 shows that even within very short time domains, the effect of the type and concentration of biocide, along with the level of organic soiling, can be quantified by using bacterial bioluminescence. The rate of decrease in bioluminescence is listed in terms of log10 RLU s−1 in Table 1. At a concentration of 1% (Fig. 2a), in the absence of organic soiling, of the four biocides tested, ECAS produced the most rapid decrease in light output (−0.379 log10 RLU s−1), followed by Virkon and bleach. One percent ethanol resulted in a small increase in emitted light. Increasing the serum concentration to 5% (Fig. 2d) and 10% (Fig. 2g) reduced the decrease in light output, and at 10% FBS the light levels of the biocides were indistinguishable from both one another and the pretreatment light level, suggesting no measurable activity within the 2-s test period.
Fig. 2.
Reduction in bioluminescence from E. coli Nissle 1917/pGLITE when exposed to bleach (○), ECAS (▼), ethanol (■), or Virkon (▵) at concentrations of 1% (a, d, and g), 10% (b, e, and h), and 80% (c, f, and i) in the absence of organic soiling (a to c) or in the presence of 5% (d to f) and 10% (g to i) fetal bovine serum. Light output is measured in RLU ± SD (n = 12).
Table 1.
Rate of change in bioluminescence of E. coli Nissle 1917/pGLITE when exposed to biocides at concentrations of 1%, 10%, and 80% under clean conditions and in the presence of 5% or 10% organic soiling
| Disinfectant type | Concn tested (%) | Rate of change in light output (log10 RLU s−1) for organic soiling (FBS) ofa: |
||
|---|---|---|---|---|
| 0% | 5% | 10% | ||
| Bleach | 1 | −0.165 ± 0.136 | −0.018 ± 0.030 | 0.076 ± 0.000 |
| 10 | −0.171 ± 0.125 | 0.001 ± 0.031 | 0.025 ± 0.020 | |
| 80 | −0.617 ± 0.070 | −0.948 ± 0.063 | −0.474 ± 0.034 | |
| ECAS | 1 | −0.379 ± 0.083 | −0.083 ± 0.021 | 0.197 ± 0.034 |
| 10 | −1.632 ± 0.263 | −0.741 ± 0.072 | 0.021 ± 0.028 | |
| 80 | −2.336 ± 0.093 | −1.579 ± 0.463 | −0.165 ± 0.051 | |
| Ethanol | 1 | 0.054 ± 0.078 | 0.0005 ± 0.029 | 0.025 ± 0.032 |
| 10 | −0.414 ± 0.133 | −0.344 ± 0.044 | −0.287 ± 0.037 | |
| 80 | −1.981 ± 0.414 | −1.938 ± 0.519 | −2.166 ± 0.188 | |
| Virkon | 1 | −0.227 ± 0.122 | −0.123 ± 0.034 | 0.040 ± 0.030 |
| 10 | −0.257 ± 0.180 | −0.082 ± 0.026 | 0.025 ± 0.032 | |
| 80 | −1.046 ± 0.163 | −0.661 ± 0.097 | −0.340 ± 0.025 | |
Data are mean RLU ± SD (n = 12).
At a biocide concentration of 10%, in the absence of FBS (Fig. 2b), the reduction in light output produced by ECAS was substantially greater (−1.632 log10 RLU s−1) than that produced by the other biocides. Ten percent ethanol produced a decrease of −0.414 log10 RLU s−1, compared to decreases of −0.257 log10 RLU s−1 and −0.171 log10 RLU s−1 for Virkon and bleach, respectively. Increasing the FBS concentration to 5% (Fig. 2e) reduced the rate of decrease in bioluminescence produced by ECAS to −0.741 log10 RLU s−1 and reduced that produced by Virkon to −0.082 log10 RLU s−1, and with bleach, it produced a slight increase in light emission over the course of the assay. At a concentration of 10% (Fig. 2b), Virkon and bleach performed similarly, despite the concentration of Virkon being below what would typically be used in practice. At a concentration of 10% and in the presence of 10% FBS, the light levels for ECAS, bleach, and Virkon were indistinguishable from each other and the pretreatment light level. Ethanol was unaffected by increasing FBS to 5%, and this was also the case using 10% FBS (Fig. 2h).
At a concentration of 80% and in the absence of FBS (Fig. 2c), ECAS produced the greatest log10 reduction and the most rapid decrease in light output (−2.336 log10 RLU s−1) of all biocides tested. Treatment with 80% ethanol was second only to treatment with ECAS in the rate and extent of reduction in bioluminescence, achieving a rate of reduction in light output of −2.166 log10 RLU s−1. In the absence of FBS, 80% Virkon produced a greater reduction in bioluminescence than 80% bleach. Adding FBS at 5% (Fig. 2f) affected all biocides with the exception of ethanol. The reduction in light output observed when treatment was with ECAS dropped to −1.579 log10 RLU s−1, and the decrease in bioluminescence produced by Virkon dropped from −1.046 to −0.661 log10 RLU s−1. Bleach produced a slightly greater reduction in light output in the presence of 5% FBS than in its absence. Further increasing the FBS to 10% reduced the decrease in bioluminescence for all biocides tested with the exception of ethanol, which was not inhibited by organic soiling. In the presence of 10% FBS, ECAS produced the smallest reduction in light output of all disinfectants tested at this concentration, at −0.165 log10 RLU s−1. The data suggest that ECAS acts very rapidly, but its activity is heavily influenced by the level of organic loading. Bleach and Virkon also had a reduced impact on bioluminescence in the presence of 10% FBS, at −0.474 and −0.340 log10 RLU s−1, respectively.
DISCUSSION
The bacterial lux system has been used extensively as a biosensor in applications ranging from assessing biocides incorporated into plastics (15) and thermal inactivation (10) to monitoring environmental pollutants using either naturally bioluminescent or genetically modified organisms (13). V. fischeri has been used as a biosensor for rapid screening of environmental samples from a wide range of sources (11). However, these systems are not routinely applied to the testing of biocides, as the time domains involved are much shorter than the 5- to 20-min exposures used for environmental toxicity testing (21). This study has demonstrated, through use of a recombinant strain expressing the full lux operon and appropriate monitoring, that bacterial bioluminescence can reveal the comparable activity of different biocides and the impact of organic soiling over time domains which would be precluded by traditional microbiological methods.
European Standard test protocols for biocides specify contact times of 5 min ± 10 s (obligatory) to a minimum of 1 min ± 10 s (additional) to assess bactericidal activity (EN 1276:1997 [5] and EN 1040:2005 [7]) or 60 min ± 10 s (obligatory) to a minimum of 5 min ± 10 s (additional) to assess sporicidal activity (EN 13704:2002 [6]). These tests, designed to assess the efficacy of biocides under practically relevant conditions, reveal little about the rate of kill of fast-acting biocides. It has been shown that novel, fast-acting biocides like ECAS can result in no recoverable bacterial survivors within 10 s (26) or immediately after mixing (9). Moreover, when used to treat bacterial spores, ECAS produced a rate of kill too fast to be accurately measured (18).
The use of the bacterial lux system reports the state of bacterial metabolism in real time. Indeed, at low concentrations of biocide or under conditions of high organic soiling, the oxygenation of the sample following injection resulted in an increase in light output throughout the assay period. This illustrates the sensitivity of the reporter strain to changing conditions. However, when challenging bioluminescent bacteria with fast-acting biocides, the emission of light is also an indicator of cell viability, as the biocides used are not limited to inhibition of metabolism but have gross effects on cellular constituents, including the lux enzymes. Ethyl alcohol causes interference with cellular metabolism but also causes membrane damage and rapid denaturation of proteins (22). Chlorine-releasing agents such as bleach are powerful oxidizing agents, with the active moiety hypochlorous acid (HClO) inactivating bacterial proteins through oxidative unfolding (34). The mechanism of activity of ECAS is not well understood, though its activity has also been attributed to the presence of HClO (16) and the contribution of a variety of oxidizing species (26). Virkon is a complex peroxygen biocide (33), and the mechanism of action of peroxygens is considered to be denaturation of cellular proteins (22).
Since all four biocides tested are capable of inactivating bacterial proteins, bacterial luciferase is a potential target, and the rate of decrease in bioluminescence acts as an indicator of the speed at which these oxidative processes take place. Perhaps most surprising are the differences observed between bleach and ECAS. Electrochemically activated water-generating systems are known to produce different active species, dependent on electrode material (16), and include HClO, free chlorine radicals, and a mixture of oxidizing substances (28). In the absence of organic soiling, ECAS at 80% resulted in the greatest reduction in bioluminescence of all biocides tested and even at 1% produced a more rapid decline in bioluminescence than 1% or 10% bleach. The ECAS produced for this study contained approximately 100-fold less free chlorine than the equivalent dilution of bleach (data not shown), suggesting that there is a contribution from reactive species other than HClO or, alternatively, that ECAS is a far more efficient chlorine-releasing agent than bleach. However, ECAS was strongly inhibited in the presence of increasing organic soiling, which is concurrent with the results of previous work utilizing traditional methods (30). Of all the biocides tested, ethanol was the least affected by organic loading. The 80% ethanol tested is within the optimum concentration range of 60 to 90% for bactericidal activity (22), and the rate of decrease in bioluminescence was second only to that of 80% ECAS (clean conditions). This activity was retained even in the presence of 10% FBS, although the main disadvantages of alcohols as biocides (lack of sporicidal activity and flammability) mean that their use is situationally limited. The overall activity of Virkon was most similar to that of bleach over the time course of the assay. Despite the dilutions to 10% and 1% (vol/vol from prepared solution) being substantially below the recommended in-use concentration for Virkon, the assay was still capable of measuring efficacy at these levels.
This work describes the use of bacterial bioluminescence in assessing the efficacy of fast-acting biocides over very short time domains. The assay can also be extended to confirm complete metabolic inhibition of the reporter, as shown in Fig. 1a. This is, however, dependent upon experimental conditions, and a full range of controls must be performed in order to establish that bacterial bioluminescence is the only source of detectable light within the system, as light-bearing reactions between certain biocides and the organic soil were detected (Fig. 1b).
This study has demonstrated that recombinant bacteria expressing the P. luminescens lux genes are capable of acting as whole-cell biosensors to report on the relative efficacies of fast-acting biocides. The bioluminescence-based assay is useful in comparing the relative efficacies of biocides immediately upon contact with bacteria and is also useful in quickly assessing the impact of organic soiling. Our findings have shown that even among biocides with similar mechanisms or with common active agents, there are substantial differences in their activity during the very early phase of kill. These differences are not measurable using traditional methodologies. The use of luminescent reporter bacteria, coupled with rapid and sensitive detection, offers a unique mechanism for monitoring the activity of biocides in real time. This assay allows the rapid comparison of the relative efficacies of fast-acting biocides immediately upon application. This has the potential to further inform our understanding of the mechanism and kinetics of microbial kill.
ACKNOWLEDGMENT
This work was funded in part by the Society for Applied Microbiology.
Footnotes
Published ahead of print on 29 August 2011.
REFERENCES
- 1. Alloush H. M., Salisbury V., Lewis R. J., MacGowan A. P. 2003. Pharmacodynamics of linezolid in a clinical isolate of Streptococcus pneumoniae genetically modified to express lux genes. J. Antimicrob. Chemother. 52:511–513 [DOI] [PubMed] [Google Scholar]
- 2. Beard S. J., Salisbury V., Lewis R. J., Sharpe J. A., MacGowan A. P. 2002. Expression of lux genes in a clinical isolate of Streptococcus pneumoniae: using bioluminescence to monitor gemifloxacin activity. Antimicrob. Agents Chemother. 46:538–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Billard P., DuBow M. S. 1998. Bioluminescence-based assays for detection and characterization of bacteria and chemicals in clinical laboratories. Clin. Biochem. 31:1–14 [DOI] [PubMed] [Google Scholar]
- 4. Bio-Rad 2002. Gene Pulser XcellTM electroporation system instruction manual. Bio-Rad, Hercules, CA [Google Scholar]
- 5. British Standards Institution 1997. BS EN 1276:1997 Chemical disinfectants and antiseptics—quantitative suspension test for the evaluation of bactericidal activity of chemical disinfectants and antiseptics used in food, industrial, domestic, and institutional areas—test method and requirements (phase 2, step 1). British Standards Institution, London, United Kingdom [Google Scholar]
- 6. British Standards Institution 2002. BS EN 13704:2002 Chemical disinfectants—quantitative suspension test for the evaluation of sporicidal activity of chemical disinfectants used in food, industrial, domestic and institutional areas—test method and requirements (phase 2, step 1). British Standards Institution, London, United Kingdom [Google Scholar]
- 7. British Standards Institution 2005. BS EN 1040:2005 Chemical disinfectants and antiseptics—quantitative suspension test for the evaluation of basic bactericidal activity of chemical disinfectants and antiseptics—test method and requirements (phase 1). British Standards Institution, London, United Kingdom [Google Scholar]
- 8. Choi S., Gu M. 1999. A whole cell bioluminescent biosensor for the detection of membrane-damaging toxicity. Biotechnol. Bioprocess Eng. 4:59–62 [Google Scholar]
- 9. Cloete T. E., Thantsha M. S., Maluleke M. R., Kirkpatrick R. 2009. The antimicrobial mechanism of electrochemically activated water against Pseudomonas aeruginosa and Escherichia coli as determined by SDS-PAGE analysis. J. Appl. Microbiol. 107:379–384 [DOI] [PubMed] [Google Scholar]
- 10. Dhir V. K., Dodd C. E. R. 1995. Susceptibility of suspended and surface-attached Salmonella enteritidis to biocides and elevated temperatures. Appl. Environ. Microbiol. 61:1731–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Doherty F. G., Qureshi A. A., Razza J. B. 1999. Comparison of the Ceriodaphnia dubia and Microtox® inhibition tests for toxicity assessment of industrial and municipal wastewaters. Environ. Toxicol. 14:375–382 [Google Scholar]
- 12. Engebrecht J., Simon M., Silverman M. 1985. Measuring gene expression with light. Science 227:1345–1347 [DOI] [PubMed] [Google Scholar]
- 13. Girotti S., Ferri E. N., Fumo M. G., Maiolini E. 2008. Monitoring of environmental pollutants by bioluminescent bacteria. Anal. Chim. Acta 608:2–29 [DOI] [PubMed] [Google Scholar]
- 14. Hill P. J., Rees C. E., Winson M. K., Stewart G. S. 1993. The application of lux genes. Biotechnol. Appl. Biochem. 17(Pt 1):3–14 [PubMed] [Google Scholar]
- 15. Jones C., Robson G. D., Greenhaulgh M., Eastwood I., Handley P. S. 2002. The development of a bioluminescence assay to compare the efficacy of biocides incorporated into plasticised PVC. Biofouling 18:21–27 [Google Scholar]
- 16. Kraft A. 2008. Electrochemical water disinfection: a short review. Platin. Met. Rev. 52:177–185 [Google Scholar]
- 17. Leon M. B., Albrecht J. A. 2007. Comparison of adenosine triphosphate (ATP) bioluminescence and aerobic plate counts (APC) on plastic cutting boards. J. Foodservice 18:145–152 [Google Scholar]
- 18. Loshon C. A., Melly E., Setlow B., Setlow P. 2001. Analysis of the killing of spores of Bacillus subtilis by a new disinfectant, Sterilox®. J. Appl. Microbiol. 91:1051–1058 [DOI] [PubMed] [Google Scholar]
- 19. Marincs F., White D. W. 1994. Immobilization of Escherichia coli expressing the lux genes of Xenorhabdus luminescens. Appl. Environ. Microbiol. 60:3862–3863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marques C. N., Salisbury V. C., Greenman J., Bowker K. E., Nelson S. M. 2005. Discrepancy between viable counts and light output as viability measurements, following ciprofloxacin challenge of self-bioluminescent Pseudomonas aeruginosa biofilms. J. Antimicrob. Chemother. 56:665–671 [DOI] [PubMed] [Google Scholar]
- 21. Martin E. B., Mansfield L. P., Smith A., Forsythe S. J. 2001. Inhibition of light emission from the bioluminescent bacterium Vibrio fischeri after exposure to triclosan and related hygiene care products. Luminescence 16:29–32 [DOI] [PubMed] [Google Scholar]
- 22. McDonnell G., Russell A. D. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 12:147–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meighen E. A. 1993. Bacterial bioluminescence: organization, regulation, and application of the lux genes. FASEB J. 7:1016–1022 [DOI] [PubMed] [Google Scholar]
- 24. Meighen E. A. 1991. Molecular biology of bacterial bioluminescence. Microbiol. Rev. 55:123–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Parveen A., Smith G., Salisbury V., Nelson S. M. 2001. Biofilm culture of Pseudomonas aeruginosa expressing lux genes as a model to study susceptibility to antimicrobials. FEMS Microbiol. Lett. 199:115–118 [DOI] [PubMed] [Google Scholar]
- 26. Robinson G. M., Lee S. W. H., Greenman J., Salisbury V. C., Reynolds D. M. 2010. Evaluation of the efficacy of electrochemically activated solutions against nosocomial pathogens and bacterial endospores. Lett. Appl. Microbiol. 50:289–294 [DOI] [PubMed] [Google Scholar]
- 27. Salisbury V., et al. 1999. Use of a clinical Escherichia coli isolate expressing lux genes to study the antimicrobial pharmacodynamics of moxifloxacin. J. Antimicrob. Chemother. 43:829–832 [DOI] [PubMed] [Google Scholar]
- 28. Shetty N., Srinivasan S., Holton J., Ridgway G. L. 1999. Evaluation of microbicidal activity of a new disinfectant: Sterilox 2500 against Clostridium difficile spores, Helicobacter pylori, vancomycin resistant Enterococcus species, Candida albicans and several Mycobacterium species. J. Hosp. Infect. 41:101–105 [DOI] [PubMed] [Google Scholar]
- 29. Siegele D. A., Hu J. C. 1997. Gene expression from plasmids containing the araBAD promoter at subsaturating inducer concentrations represents mixed populations. Proc. Natl. Acad. Sci. U. S. A. 94:8168–8172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tanaka H., et al. 1996. Antimicrobial activity of superoxidized water. J. Hosp. Infect. 34:43–49 [DOI] [PubMed] [Google Scholar]
- 31. Tanner R. S. 1989. Comparative testing and evaluation of hard-surface disinfectants. J. Ind. Microbiol. 4:145–154 [Google Scholar]
- 32. Thorn R. M., Nelson S. M., Greenman J. 2007. Use of a bioluminescent Pseudomonas aeruginosa strain within an in vitro microbiological system, as a model of wound infection, to assess the antimicrobial efficacy of wound dressings by monitoring light production. Antimicrob. Agents Chemother. 51:3217–3224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Walker A. J., Holah J. T., Denyer S. P., Stewart G. S. 1992. The antibacterial activity of Virkon measured by colony growth and bioluminescence of lux recombinant Listeria monocytogenes. Lett. Appl. Microbiol. 15:80–82 [DOI] [PubMed] [Google Scholar]
- 34. Winter J., Ilbert M., Graf P. C., Ozcelik D., Jakob U. 2008. Bleach activates a redox-regulated chaperone by oxidative protein unfolding. Cell 135:691–701 [DOI] [PMC free article] [PubMed] [Google Scholar]