Abstract
Background:
Some individuals experience a discordant response during antiretroviral therapy (ART), with a blunted CD4+ cell count response despite low HIV-1 RNA plasma levels.
Materials and Methods:
CD4 counts and viral load of 251 individuals on ART referred to the center were analysed for immunological failure. The viral load tests of 28 patients revealed a discordant response, characterized by low CD4 counts despite viral suppression (<47 copies in 23, <5000 in 4 patients and <10000 in one patient). Univariate and multiple regression analysis was done to determine factors associated with immunological failure in patients with viral suppression.
Results:
Twenty-eight patients developed immunological failure over a duration of 3.7±1.14 years despite viral suppression. In univariate analysis of discordant patients, low CD4 counts(<100cells/μl) at start of ART(P=0.0261), less than 50% gain in CD4 count (P=0.048) after one year of start of ART and duration on ART for more than 3 years (P=0.0436) were associated with immunological failure. In multiple regression, duration on ART, age and nadir CD4 count (lowest ever) on treatment were predictors of immunological failure in these patients. Overall females (n=8) demonstrated much higher CD4 counts of 136±72 than males (n=20) 79±38 cells/μl at the time of diagnosis of immunological failure.
Conclusions:
Discordance was observed in 13.59% of patients. Detection of failure to first line therapy based on immunologic criteria, without viral load testing, can result in unnecessary switches to 2nd line therapy.
Keywords: Antiretroviral therapy, discordant response, immunological failure, viral load, viral suppression
INTRODUCTION
The use of highly active antiretroviral therapy (HAART) has improved the survival of patients infected with human immunodeficiency virus (HIV), slowing the progression of the infection towards acquired immunodeficiency syndrome (AIDS). This is achieved through an immunological recovery with increase of CD4 T-cell count and decrease in viral load to undetectable levels.[1–2] Viral load and CD4 T-cell counts are the most commonly used parameters to monitor the efficiency of antiretroviral treatment.[3] Because of the association observed between immunologic and virologic responses in the first months of treatment, it is a common practice to follow-up these parameters to determine therapeutic success or failure and evaluate changes in antiretroviral treatment in early stages.[4–7] There are groups of patients where viral replication is suppressed appropriately but without immunologic recovery. On the other hand, there are patients with immunologic recovery but without an important decrease in viral load. These two scenarios are known as discordant responses.[8–12]
In this study cohort on ART, characteristics of virological responders, with poor CD4 T cell response, were studied to determine factors that may predict discordance.
MATERIALS AND METHODS
This cross-sectional study was conducted at ART Centre of Bowring and Lady Curzon Hospital (Bangalore Medical College and Research Institute), Bangalore. Permission was obtained by the Karnataka State AIDS Prevention Society for conducting this study and publishing this data. Patients receive standardized first-line anti retro-viral (ARV) regimen of Zidovudine (AZT)/Lamivudine (3TC) or Stavudine (D4T)/3TC + Nevirapine (NVP) or Efavirenz (EFV). Patients are seen monthly for clinical assessment, adherence, counseling and drug pick up. CD4 cell count is performed every 6 months; viral load testing is not performed routinely due to economical constraints in resource poor settings.
Adult and pediatric patients who have received 2 years or more of standard first-line ART are referred for second-line ART evaluation if they demonstrated CD4 decline to pre-ART values (baseline-at start of ART), CD4 drop to less than 50% of peak on-treatment value, failure to achieve CD4 greater than 100 c/mm3. Patients received HIV RNA testing, and those with HIV RNA 10,000 copies/mL or greater qualified to switch to second-line ART.
Patients with immunological failure were referred to be evaluated by State AIDS Clinical Expert Panel (SACEP). During the process of evaluation, we found patients having viral load below detectable levels (<47 copies/ml) and few with viral load copies <10,000 copies/ml despite immunological failure. These discordant patients were selected for the purpose of analysis in this study.
RESULTS
Of the 251 patients referred for second-line ART, 206 underwent estimation of viral load. Twenty eight (13.59%) patients showed a “discordant response” whereby HIV-1 RNA plasma level is below the limit of detection but the CD4+ cell count response is blunted. These discordant patients were referred from different district ART Centers of Karnataka based on the criteria of immunological failure, i.e., if they demonstrated CD4 decline to pre-ART values (baseline-at start of ART), CD4 drop to less than 50% of peak on-treatment value, failure to achieve CD4 greater than 100 c/mm3. to be further evaluated by SACEP. Viral copies were below detectable levels (<47 copies /ml) in 23 patients, less than 5000 c/ml in 4 patients, and 1 patient had less than 10,000 copies/ml with low CD4 counts.
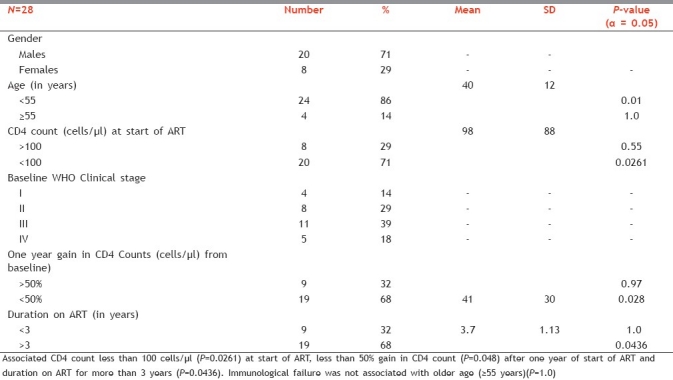
Among them, 20 (71%) were males (which included one pediatric patient) and 8 (29%) were females. Median age was 38.5 years (interquartile range: 35-44). Mean time to failure for patients with immunologic failure without virologic failure was 3.7±1.14 years. Mean CD4 count at the time of start of ART and at the time of referral to SACEP as a result of immunological failure was 98 ± 88 and 95 ± 55 cells/μl, respectively as shown in Table 1.
Table 1.
Results of univariate analysis performed on 28 immunologic non responders despite viral suppression showing immunologic failure
Overall females demonstrated much higher CD4 counts of 136 ± 72 than males 79 ± 38 at the time of diagnosis of immunological failure [Figure 1].
Figure 1.
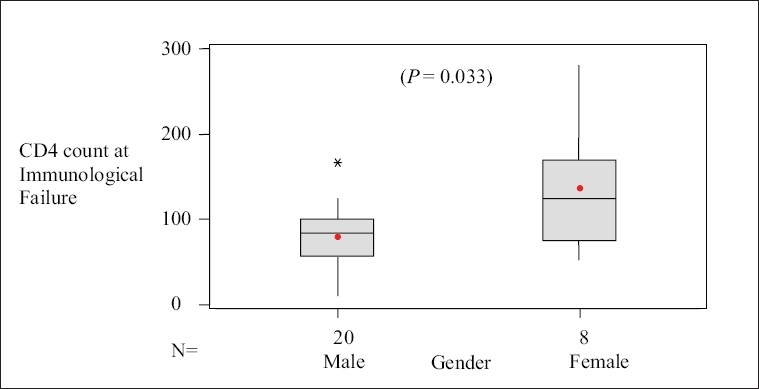
Comparison of CD4 count at the time of immunological failure in male and female discordant patients. Boxes represent interquartile range, horizontal line inside box is median value. Means are indicated by solid circles
Out of these 28 discordant patients, 9 (32%) were referred to SACEP as they demonstrated a decline of CD4 counts to below baseline values, 11 (39%) patients had CD4 drop to less than 50% of peak on-treatment value and 8 (29%) failed to achieve more than 100 cells/μl. The median nadir (highest ever) CD4 counts ever achieved by these patients was 165 (range: 19–576) and nadir (lowest ever) was 66 (range: 6-172) cells/μl. According to National guidelines only those patients with HIV RNA 10,000 copies/ml or greater are qualified to receive second-line ART under the National program. Since the patients were asymptomatic and had optimal viral suppression on ART and as there are no currently available guidelines to specifically treat these discordant patients, the patients had to be deferred back to their Nodal ART Centers to continue on their current regimen. Twenty (71%) were currently on Zidovudine-based regimen and 8(29%) were on Stavudine-based regimen. Adherence history of these patients showed more than 95% adherence in NACO recognized government ART centers. The history of 5 patients showed receipt of ART at private clinics. There was discontinuity in taking ART in these patients due to economic problems after which they had been started ART at government ART centers. Adverse effects to ART were noted in 3 patients with peripheral neuropathy, 3 patients with anemia, and 5 patients with lipodystrophy.
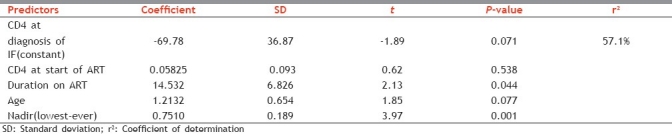
In Multiple Regression analysis, we examined those variables that showed a degree of association in univariate analysis (P<0.05). The regression equation is CD4 current = - 69.8 + 0.0583 CD4 at start + 14.5 Duration on ART (in yrs) + 1.21 Age + 0.751 Nadir (lowest ever) on Rx CD4
From Table 2, duration on ART, age, and nadir (lowest ever) on treatment CD4 count were predictors of immunological failure in these patients.
Table 2.
Regression analysis
DISCUSSION
In 2008, NACO piloted a national strategy for the provision of free second-line ART in India. Patients demonstrating WHO-defined immunologic or clinical treatment failure are evaluated by expert panel and then are recommended for HIV RNA testing to confirm virologic failure.
The initiation of HAART generally leads to a rapid reduction in HIV-1 RNA plasma levels and to an increase in peripheral CD4+ cell counts.[13–15] However, some patients experience a “discordant response”, whereby the HIV-1 RNA plasma level is below the limit of detection but the CD4+ cell count response is blunted. There are limited data on discordant responses in patients being treated in India.
A study, the Antiretroviral Therapy in Lower Income Countries Collaboration (ART-LINC), an epidemiological network of HIV/AIDS treatment programmes in Africa, Asia and South America, has reported on the frequency of discordant responses seen in 15 developing countries. A total of 269 patients (14%) were virological only responders, with virological response defined as achieving a plasma HIV RNA viral load <500 copies/mL.[16] In our study 28 patients (24%) out of 116 who had their viral load tests done were found to be virological only responders.
In developing countries, clinical evolution, viral load and CD4 T-cell count follow-up are the only tools available to physicians for evaluation of treatment efficiency.[4,17] Developed countries began to analyze HIV genotypes searching for mutations associated with antiretroviral resistance in patients who presented treatment failure. This practice is expanding to patients without previous treatment, with the purpose of providing “customized” treatment from the beginning and reduce the risk of therapeutic failure.[18] Most HIV seropositives reside in developing countries. With limited facilities and high costs genotype studies are limited.
Little is known about the pathogenesis of discordant responses, which seems to depend on the interaction of a multitude of viral, host, and treatment-related factors. Several studies have found that genetic polymorphisms, including the Fas receptor (CD95) gene, the Fas ligand (CD178), the IL-6 gene, and the MHC genes are involved in T-cell immunity and affect whether an individual experiences an immunologic response to HAART therapy or not.[19,20] A blunted CD4 response despite suppression of viral replication has often been attributed to host characteristics, particularly older age.[7,21–24] It has been hypothesized that the magnitude of immune restoration is dependent on thymus activity, which decreases with age. Our study found that maximum (86%) of discordant patients were <55 years of age compared to individuals >55 years of age. (P=1.0). Virological response with immunological failure was associated age <55 years (P=0.01).
This study demonstrated that a CD4 count was <100 cells at start of ART. Our findings were similar to the findings of other studies, which suggest that poor CD4 cell reconstitution despite virological response has been correlated with a lower nadir pretreatment CD4+ cell count, suggestive of more extensive depletion of CD4+ cells in the gut-associated lymphoid tissue during primary (acute) HIV infection, which may be slow or refractory to reconstitution with antiretroviral therapy.[25,26]
In a study by Onen et al which examined risk factors for sub-optimal CD4 recovery on suppressive highly active antiretroviral therapy (HAART) found that 36% patients had suboptimal (<150 cells/μl) during the first year of virological suppression.[27] In our study, 68% (P=0.028) of the virological only responders had CD4 recovery of less than 50% from the baseline value during first year of therapy. Also, it was found that duration of more than 3 years on ART was significantly associated with immunological failure.
The use of zidovudine as part of the antiretroviral regimen have been associated with suboptimal CD4+ cell responses despite suppression of viral replication.[28] In our study, 71% of virological only responders were currently on Zidovudine-based regimen and 29% were on Stavudine-based regimen. Adherence to therapy may also influence the occurrence of discordant responses. In the study by Moore et al., suboptimal adherence was found to be associated both with virological and immunological responses.[21]
CONCLUSIONS
Discordant response was noted in 13.59% of patients referred for viral load testing based on immunologic criteria. Thus, immunological failure should not be interpreted as failure to first-line ART without considering viral load test. This can result in unnecessary switches to second-line therapy.
Since CD4 count less than 100 cells/μl (P=0.0261) at start of ART was found to be a risk factor for immunologic failure, it is necessary to ensure and monitor strict adherence to ART in those patients starting at very low CD4 counts through counseling.
ACKNOWLDGEMENTS
We would like to thank National AIDS Control Organisation (NACO) and Karnataka State AIDS Prevention Society (KSAPS), Dean and Director, BMCRI and Superintendent, Bowring and Lady Curzon Hospital for their support in conducting this study.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Starr SE, Fletcher CV, Spector SA, Yong FH, Fenton T, Brundage RC, et al. Combination therapy with efavirenz, nelfinavir, and nucleoside reverse-transcriptase inhibitors in children infected with human immunodeficiency virus type 1. Pediatric AIDS Clinical Trials Group 382 Team. N Engl J Med. 1999;341:1874–81. doi: 10.1056/NEJM199912163412502. [DOI] [PubMed] [Google Scholar]
- 2.Staszewski S, Morales-Ramirez J, Tashima KT, Rachlis A, Skiest D, Stanford J, et al. Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. Study 006 Team. N Engl J Med. 1999;341:1865–73. doi: 10.1056/NEJM199912163412501. [DOI] [PubMed] [Google Scholar]
- 3.Working Group on Antiretroviral Therapy and Medical Management of HIV-infected children, United States. Health Resources and Services Administration. National Institutes of Health (U.S.). Guidelines for the use of antiretroviral agents in pediatric HIV infection. Bethesda, MD: National Institutes of Health; 2008. Jun 28, Health Resources and Services Administration. [Google Scholar]
- 4.Mellors JW, Muñoz A, Giorgi JV, Margolick JB, Tassoni CJ, Gupta P, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–54. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien WA, Hartigan PM, Daar ES, Simberkoff MS, Hamilton JD. Changes in plasma HIV RNA levels and CD4+ lymphocyte counts predict both response to antiretroviral therapy and therapeutic failure. VA Cooperative Study Group on AIDS. Ann Intern Med. 1997;126:939–45. doi: 10.7326/0003-4819-126-12-199706150-00002. [DOI] [PubMed] [Google Scholar]
- 6.Mehta SH, Lucas G, Astemborski J, Kirk GD, Vlahov D, Galai N. Early immunologic and virologic responses to highly active antiretroviral therapy and subsequent disease progression among HIV-infected injection drug users. AIDS Care. 2007;19:637–45. doi: 10.1080/09540120701235644. [DOI] [PubMed] [Google Scholar]
- 7.Nicastri E, Chiesi A, Angeletti C, Sarmati L, Palmisano L, Geraci A, et al. Clinical outcome after 4 years follow-up of HIV-seropositive subjects with incomplete virologic or immunologic response to HAART. J Med Virol. 2005;76:153–60. doi: 10.1002/jmv.20352. [DOI] [PubMed] [Google Scholar]
- 8.Ghaffari G, Passalacqua DJ, Caicedo JL, Goodenow MM, Sleasman JW. Two-year clinical and immune outcomes in human immunodeficiency virus-infected children who reconstitute CD4 T cells without control of viral replication after combination antiretroviral therapy. Pediatrics. 2004;114:604–11. doi: 10.1542/peds.2004-0274. [DOI] [PubMed] [Google Scholar]
- 9.Flynn PM, Rudy BJ, Douglas SD, Lathey J, Spector SA, Martinez J, et al. Virologic and immunologic outcomes after 24 weeks in HIV type 1-infected adolescents receiving highly active antiretroviral therapy. J Infect Dis. 2004;190:271–9. doi: 10.1086/421521. [DOI] [PubMed] [Google Scholar]
- 10.Sufka SA, Ferrari G, Gryszowka VE, Wrin T, Fiscus SA, Tomaras GD, et al. Prolonged CD4+ cell/virus load discordance during treatment with protease inhibitor-based highly active antiretroviral therapy: Immune response and viral control. J Infect Dis. 2003;187:1027–37. doi: 10.1086/368359. [DOI] [PubMed] [Google Scholar]
- 11.Dronda F, Moreno S, Moreno A, Casado JL, Pérez-Elías MJ, Antela A. Long-term outcomes among antiretroviral-naive human immunodeficiency virus-infected patients with small increases in CD4+ cell counts after successful virologic suppression. Clin Infect Dis. 2002;35:1005–9. doi: 10.1086/342695. [DOI] [PubMed] [Google Scholar]
- 12.D’Ettorre G, Forcina G, Andreotti M, Sarmati L, Palmisano L, Galluzzo CM, et al. Discordant response to antiretroviral therapy: HIV isolation, genotypic mutations, T-cell proliferation and cytokine production. AIDS. 2002;16:1877–85. doi: 10.1097/00002030-200209270-00004. [DOI] [PubMed] [Google Scholar]
- 13.Gulick RM, Mellors JW, Havlir D, Eron JJ, Gonzalez C, McMahon D, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–9. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 14.Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, Currier JS, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337:725–33. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 15.Montaner JS, Reiss P, Cooper D, Vella S, Harris M, Conway B, et al. A randomized, double-blind trial comparing combinations of nevirapine, didanosine, and zidovudine for HIV-infected patients: the INCAS Trial.Italy, The Netherlands, Canada and Australia Study. JAMA. 1998;279:930–7. doi: 10.1001/jama.279.12.930. [DOI] [PubMed] [Google Scholar]
- 16.Schechter M, Brinkhof M, Egger M, May M, Nash D, Sprinz E, et al. Alexandra, VA, USA: Foundation for Retrovirology and Human Health; 2006. Discordant immunologic and virologic responses to ART among previously naive adults initiating HAART in resource-constrained settings. In: Programs and Abstracts of the Thirteenth Conference on Retroviruses and Opportunistic Infections, Denver; p. 229. Abstract 559. [Google Scholar]
- 17.Resino S, Resino R, Leon JA, Bellon JM, Martin-Fontelos P, Ramos JT, et al. Impact of longterm viral suppression in CD4+ recovery of HIVchildren on highly active antiretroviral therapy. BMC Infect Dis. 2006;6:10. doi: 10.1186/1471-2334-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Bethesda, MD: National Institutes of Health; 2008. Nov 3, National Institutes of Health (U.S.). Office of AIDS Research. Advisory Council, United States. Public Health Service. [Google Scholar]
- 19.Nasi M, Pinti M, Bugarini R, Troiano L, Lugli E, Bellodi C, et al. Genetic polymorphisms of Fas (CD95) and Fas ligand (CD178) influence the rise in CD4+ T cell count after antiretroviral therapy in drug-naive HIV-positive patients. Immunogenetics. 2005;57:628–35. doi: 10.1007/s00251-005-0031-z. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez S, Rosenow AA, James IR, Roberts SG, Nolan RC, French MA, et al. Recovery of CD4+ T cells in HIV patients with a stable virologic response to antiretroviral therapy is associated with polymorphisms of interleukin-6 and central major histocompatibility complex genes. J Acquir Immune Defic Syndr. 2006;41:1–5. doi: 10.1097/01.qai.0000188990.57760.e3. [DOI] [PubMed] [Google Scholar]
- 21.Moore DM, Hogg RS, Yip B, Wood E, Tyndall M, Braitstein P, et al. Discordant immunologic and virologic responses to highly active antiretroviral therapy are associated with increased mortality and poor adherence to therapy. J Acquir Immune Defic Syndr. 2005;40:288–93. doi: 10.1097/01.qai.0000182847.38098.d1. [DOI] [PubMed] [Google Scholar]
- 22.Kaufmann GR, Furrer H, Ledergerber B, Perrin L, Opravil M, Vernazza P, et al. Characteristics, determinants, and clinical relevance of CD4 T cell recovery to <500 cells/microL in HIV type 1-infected individuals receiving potent antiretroviral therapy. Clin Infect Dis. 2005;41:361–72. doi: 10.1086/431484. [DOI] [PubMed] [Google Scholar]
- 23.Piketty C, Weiss L, Thomas F, Mohamed AS, Belec L, Kazatchkine MD. Long-term clinical outcome of human immunodeficiency virus-infected patients with discordant immunologic and virologic responses to a protease inhibitor-containing regimen. J Infect Dis. 2001;183:1328–35. doi: 10.1086/319861. [DOI] [PubMed] [Google Scholar]
- 24.Marimoutou C, Chêne G, Mercié P, Neau D, Farbos S, Morlat P, et al. Prognostic factors of combined viral load and CD4+ cell count responses under triple antiretroviral therapy, Aquitaine cohort, 1996-1998. J Acquir Immune Defic Syndr. 2001;27:161–7. doi: 10.1097/00126334-200106010-00011. [DOI] [PubMed] [Google Scholar]
- 25.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–59. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Douek DC, Picker LJ, Koup RA. T cell dynamics in HIV-1 infection. Annu Rev Immunol. 2003;21:265–304. doi: 10.1146/annurev.immunol.21.120601.141053. [DOI] [PubMed] [Google Scholar]
- 27.Onen NF, Overton ET, Presti R, Blair C, Powderly WG, Mondy K. Sub-optimal CD4 recovery on long-term suppressive highly active antiretroviral therapy is associated with favourable outcome. HIV Med. 2009;10:439–46. doi: 10.1111/j.1468-1293.2009.00711.x. [DOI] [PubMed] [Google Scholar]
- 28.US Department of Health and Human Services. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. [last accessed on 2006 June 13]. Available from: http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescent GL.pdf .


