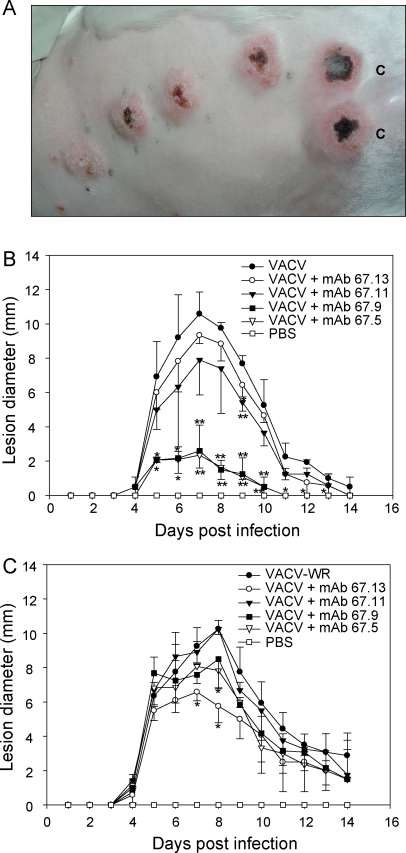
Fig. 6.
Monoclonal antibody effect in vivo in a rabbit skin lesion VACV-WR challenge model. (A) Skin lesions developed after intradermal injection of 104 pfu of VACV-WR (extreme right lesions marked as C) or 104 pfu of virus mixed with graded concentrations of mAb 67.5 (25 μg, 12.5 μg, 6.3 μg and 3 μg, first four lesions from left). The image was taken 8 days post infection. (B) Skin lesion size after intradermal injection of 104 pfu of VACV-WR alone or in combination with indicated antibodies (25 μg) in rabbits. (C) Skin lesion size after intradermal injection of 104 pfu of VACV-WR alone or mixed with 25 μg of the indicated antibodies in rabbits depleted of complement by injecting cobra venom factor 6 h prior to infection. Data points presented in panels (B and C) represent mean of four replicates performed in two rabbits. Statistical comparisons were performed between mAb treated lesions and untreated lesions. *p < 0.01 and **p < 0.001 (Mann–Whitney Rank Sum test, SigmaStat). Differences are also significant between 67.13 treated lesions and 67.5/67.11 treated lesions between days 5 and 11 (p ≤ 0.001).