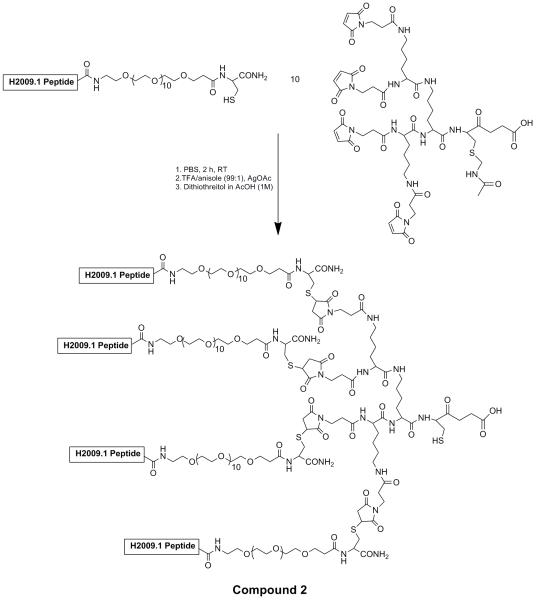
Scheme II. Synthesis of tetrameric H2009 peptide by a convergent coupling reaction (compound 2).
Michael addition of cysteine containing monomeric peptides with the maleimide trilysine core results in formation of the tetrameric peptide. A PEG11 linker is placed between the peptide and the core to improve solubility and prevent peptide aggregation. Removal of the acetamidomethyl protein group from the trilysine core by treatment with the Ag(OAc) reveals a unique thiol for coupling to compound 1.