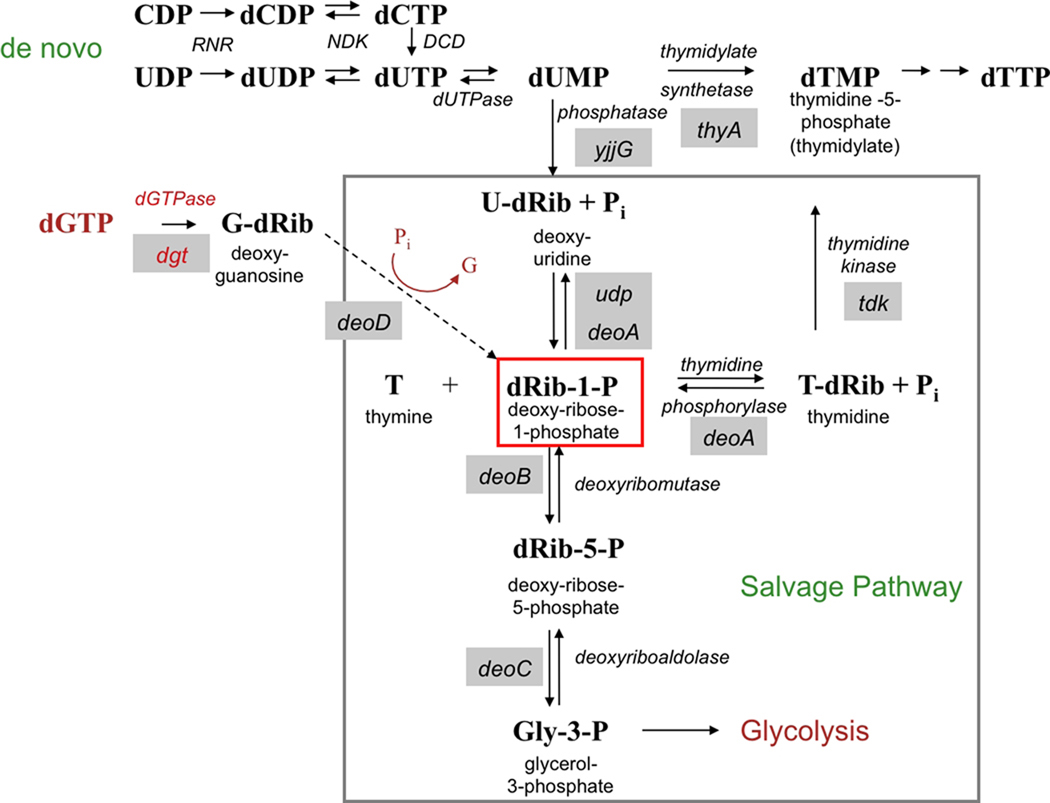
Fig. 1.
de novo and salvage pathways for thymidylate synthesis (adapted from Zaritsky et al., 2006) as present in E. coli. thyA mutants cannot produce thymidylate by the de novo route [mediated by ribonucleotide reductase (RNR), nucleotide diphosphate kinase (NDK) and dCTP deaminase (DCD)] but, in contrast to wild-type cells, they have increased potential for using intra- and extracellular thymine for DNA incorporation. The mechanism involves accumulation of deoxyribose-1-phosphate (dRib-1-P) (central red square) because of increased dUMP hydrolysis due to the thyA block. dUMP phosphatase (encoded by yjjG) converts dUMP to deoxyuridine (U-dRib), which undergoes phosphorolysis to U and dRib-1-P by uridine or thymidine phosphorylase (udp or deoA gene, respectively). dRib-1-P can condense with thymine to generate thymidine in a reaction catalyzed by thymidine phosphorylase (encoded by deoA). Deoxyguanosine (the direct product of the reaction catalyzed by dGTPase (produced by the dgt gene) can further enlarge the dRib-1-P pool through purine nucleoside phosphorylase activity encoded by deoD, thus enhancing thymine incorporation.