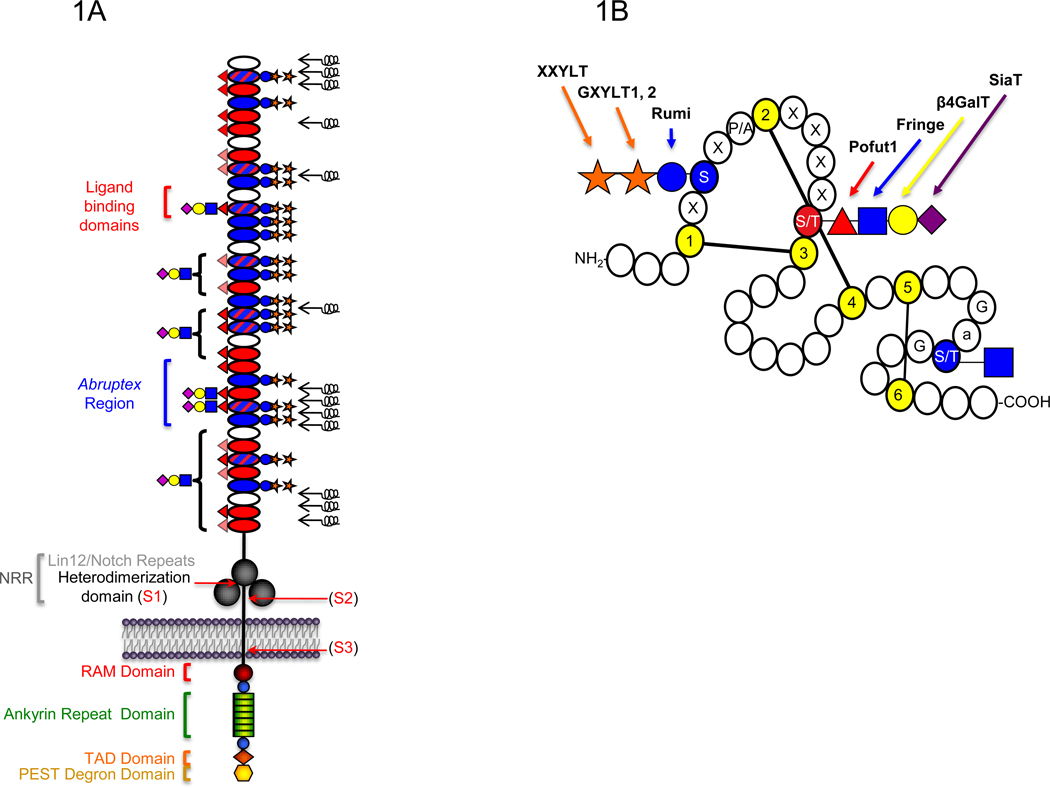
Figure 1. Schematic representation of the domain structure of the Notch receptor and an Epidermal Growth Factor-like (EGF) repeat.
A. The extracellular domain (ECD) of mouse Notch1 consists of 36 tandem EGF repeats (ovals) followed C-terminally by three Lin12/Notch repeats (LNRs) (large grey circles) and a heterodimerization domain (black line) [1]. EGF repeats containing the consensus sequence for O-glucosylation, O-fucosylation, or both, are filled with blue, red, or blue and red hatched lines, respectively. Mapped O-fucose and O-glucose glycans are indicated on each EGF repeat based on published data [19–21] (lightly shaded red triangles indicate O-fucose sites not yet confirmed). Results suggest that all O-glucose sites are modified with O-glucose trisaccharide, although some O-glucose monosaccharide form also exists at lower levels (not shown) [19]. Elongation of the saccharide beyond the O-fucose is based on work from [20,21]. Brackets indicate that elongation occurs on some or all of the O-fucose sites in that region. Symbols are based on Consortium for Functional Glycomics guidelines: glucose, blue circle; xylose, orange star; fucose, red triangle; GlcNAc, blue square; Galactose, yellow circle; Sialic Acid, purple diamond. Non-calcium-binding EGF repeats are unable to coordinate calcium ions to their N-terminal linker region, and are predicted to be flexible regions of the ECD (black springs) [47]. Ligands are known to physically interact with EGF11–12 [44,45]. The Abruptex region is defined by a series of mutations in EGF 24–29 of Drosophila Notch known to hyperactivate Notch and be refractory to Fringe [43]. The LNRs and heterodimerization domain together form the Negative Regulatory Region (NRR). Recent structural studies suggest that in the resting state, the LNRs mask the heterodimerization domain, preventing proteolysis [49,50]. Ligand binding is believed to result in a conformational change exposing the S2 protease site (see Figure 2). Sites of receptor cleavage events (S1-S3) are indicated with red arrows. The intracellular domain (ICD) is rich in several regulatory motifs as indicated, including a RAM (Regulation of Amino Acid Metabolism) domain, immediately followed by a Nuclear Localization Signal (NLS) (blue circles) and seven tandem ankyrin repeats (green rectangles). Following these motifs are a second NLS (blue circle), a Transcriptional Activation Domain (TAD) (orange diamond), and a PEST (Pro, Glu, Ser, Thr-rich) degron sequence involved in turnover of the ICD at the C-terminus (yellow hexagon). B. Short 40 amino acid motifs known as EGF repeats are defined by the presence of six conserved Cys (yellow) that form three disulfide bridges. Potential sites of O-glucosylation (C1-x-S-x-P/A-C2), O-fucosylation (C2-x-x-x-x-S/T-C3), and O-GlcNAc modification (no consensus sequence defined, but observed between Cys 5 and Cys 6 on Ser or Thr following Gly and an aromatic residue (a), and preceding Gly) are indicated in red or blue. Glycosyltransferases that carry out addition of each sugar are indicated with arrows.