Figure 2.
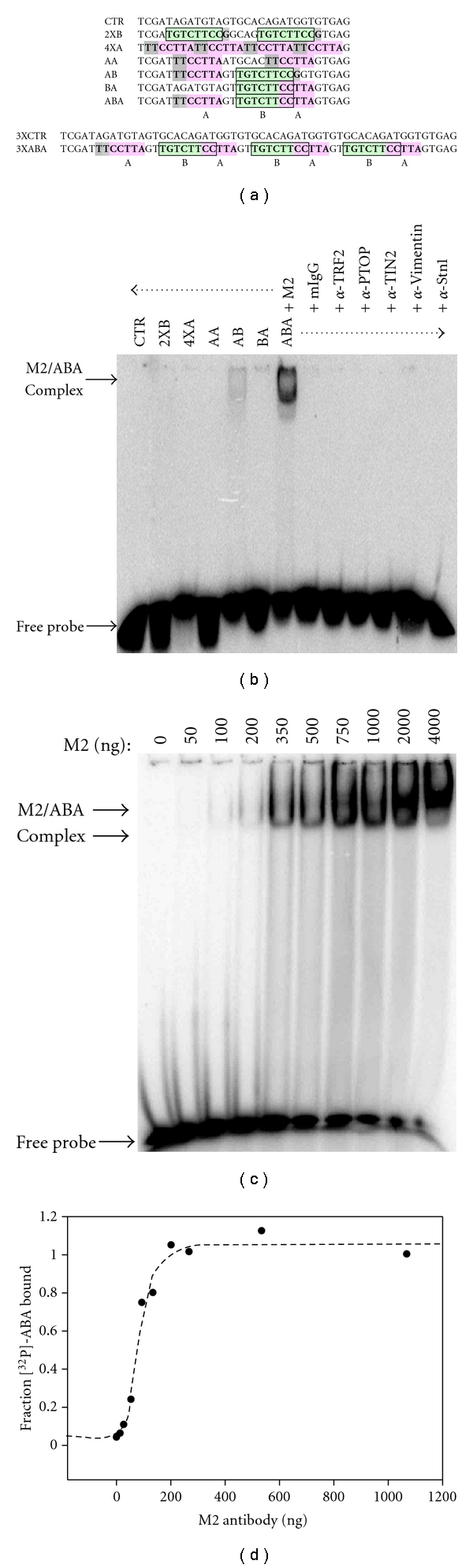
The bipartite ssDNA consensus binds directly to the anti-Flag M2 antibody. (a) Sequence and graphical representation of the probes used. All probes were labeled at the 5′-end with [32P]. Probes were designed to carry none, one, or both of the identified motifs. Motif A = CCTTA (pink). Motif B = TGTCTWCC (green). (b) The anti-Flag M2 antibody binds selectively to probe ABA. The indicated probes (80,000 cpm) were incubated with the listed antibodies (1 μg), after which protein/DNA complexes were resolved by electrophoresis in a native polyacrylamide gel. (c) Titration of the M2 antibody. A constant amount of probe ABA was incubated with an increasing concentration of M2 antibody, and the protein/DNA complexes were resolved by native electrophoresis. At the higher antibody concentrations, a larger protein/DNA complex is observed (top arrow), which may represent the product of antibody oligomerization. (d) Binding isotherm of probe ABA interacting with the anti-Flag M2 antibody. The scatter plot shows the amount of ABA bound (amount present in both protein/DNA complexes) as a function of antibody concentration. Dotted line shows fitting of the data to a “one site” saturation binding curve. Nonlinear regression of the data allowed for the determination of the dissociation constant (KD = 80 ± 7 nM). Error on the value of the KD represents the 95% confidence interval of the best-fit curve.
